Unknown Story
Öykü Penceresi Metni
- l
- i
- i
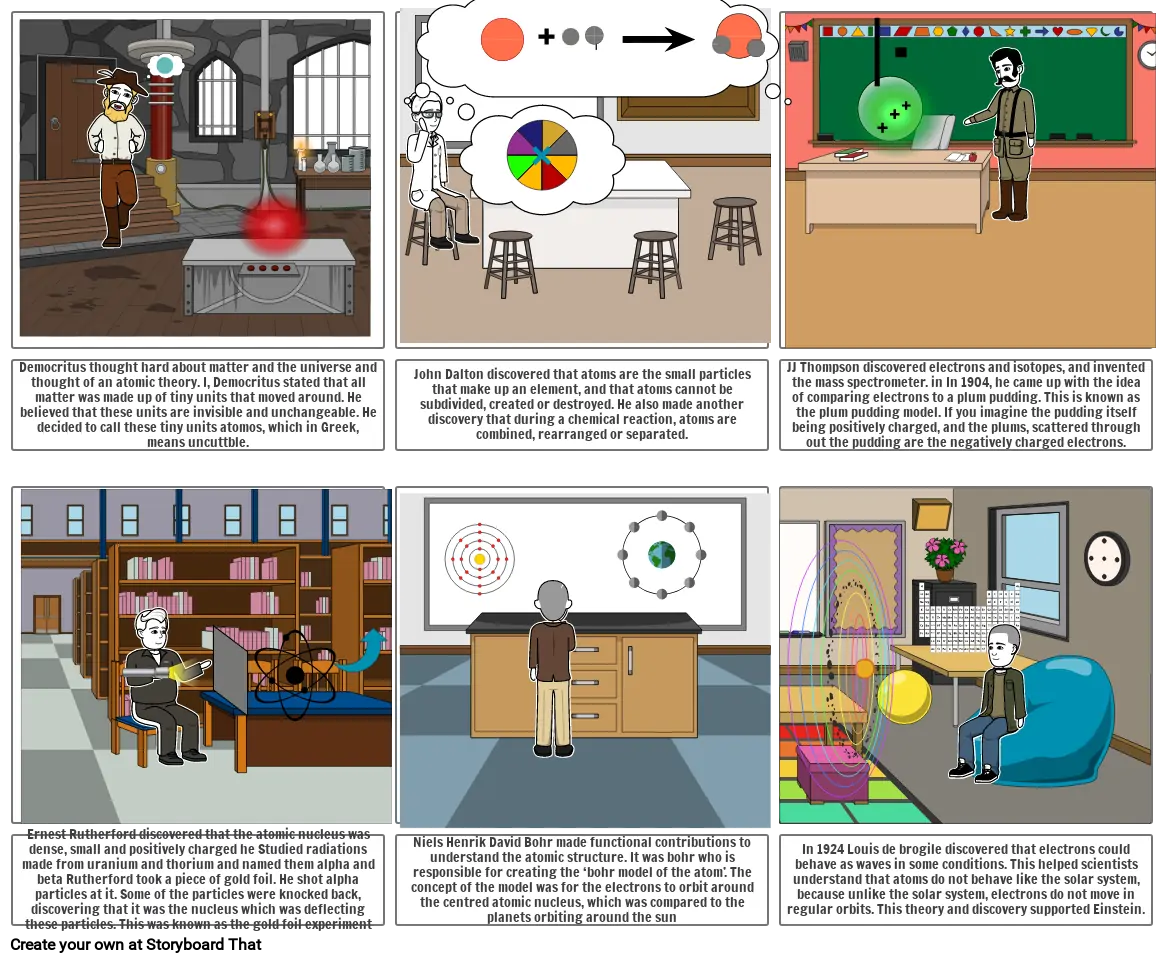
- Democritus thought hard about matter and the universe and thought of an atomic theory. I, Democritus stated that all matter was made up of tiny units that moved around. He believed that these units are invisible and unchangeable. He decided to call these tiny units atomos, which in Greek, means uncuttble.
- John Dalton discovered that atoms are the small particles that make up an element, and that atoms cannot be subdivided, created or destroyed. He also made another discovery that during a chemical reaction, atoms are combined, rearranged or separated.
- JJ Thompson discovered electrons and isotopes, and invented the mass spectrometer. in In 1904, he came up with the idea of comparing electrons to a plum pudding. This is known as the plum pudding model. If you imagine the pudding itself being positively charged, and the plums, scattered through out the pudding are the negatively charged electrons.
- Ernest Rutherford discovered that the atomic nucleus was dense, small and positively charged he Studied radiations made from uranium and thorium and named them alpha and beta Rutherford took a piece of gold foil. He shot alpha particles at it. Some of the particles were knocked back, discovering that it was the nucleus which was deflecting these particles. This was known as the gold foil experiment
- Niels Henrik David Bohr made functional contributions to understand the atomic structure. It was bohr who is responsible for creating the ‘bohr model of the atom’. The concept of the model was for the electrons to orbit around the centred atomic nucleus, which was compared to the planets orbiting around the sun
- In 1924 Louis de brogile discovered that electrons could behave as waves in some conditions. This helped scientists understand that atoms do not behave like the solar system, because unlike the solar system, electrons do not move in regular orbits. This theory and discovery supported Einstein.
30 Milyondan Fazla Storyboard Oluşturuldu

