Boyle's Law
Öykü Penceresi Metni
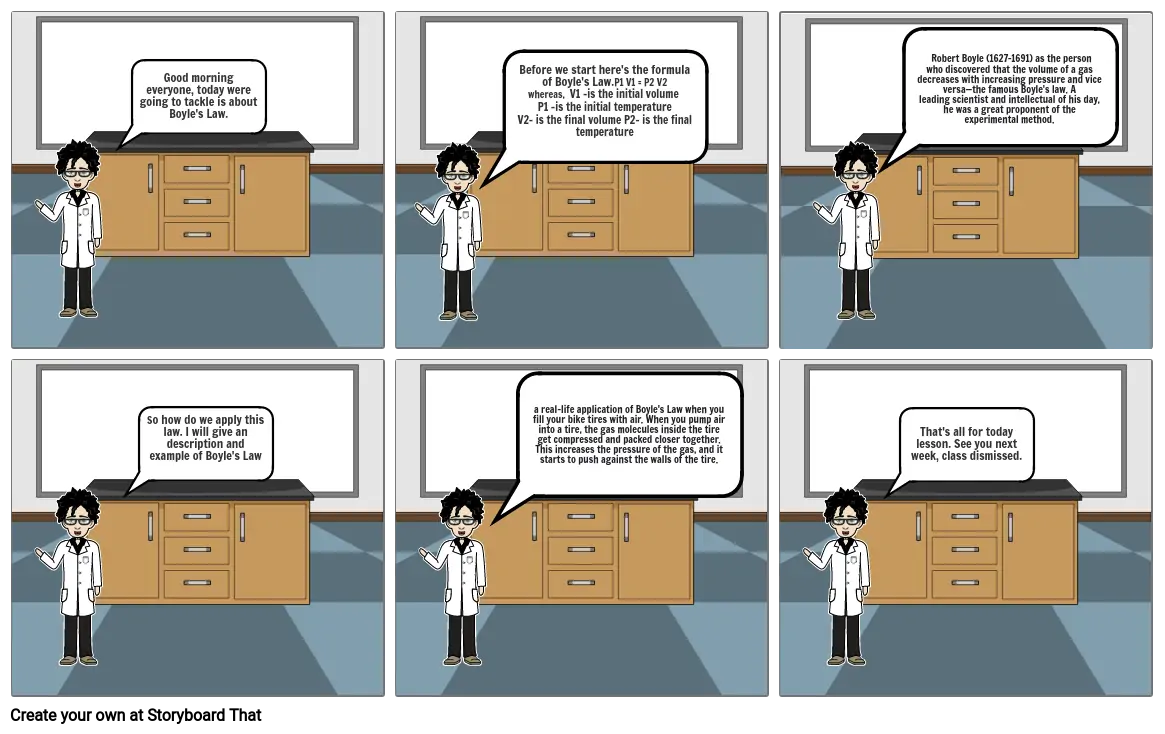
- Good morning everyone, today were going to tackle is about Boyle's Law.
- Before we start here's the formula of Boyle's Law.P1 V1 = P2 V2whereas, V1 -is the initial volume P1 -is the initial temperatureV2- is the final volume P2- is the final temperature
- Robert Boyle (1627–1691) as the person who discovered that the volume of a gas decreases with increasing pressure and vice versa—the famous Boyle's law. A leading scientist and intellectual of his day, he was a great proponent of the experimental method.
- So how do we apply this law. I will give an description and example of Boyle's Law
- a real-life application of Boyle's Law when you fill your bike tires with air. When you pump air into a tire, the gas molecules inside the tire get compressed and packed closer together. This increases the pressure of the gas, and it starts to push against the walls of the tire.
- That's all for today lesson. See you next week, class dismissed.
30 Milyondan Fazla Storyboard Oluşturuldu

