History of the Atomic Theory (Continued)
(The most important conclusion or discovery that each scientist made is in the speech bubble.)
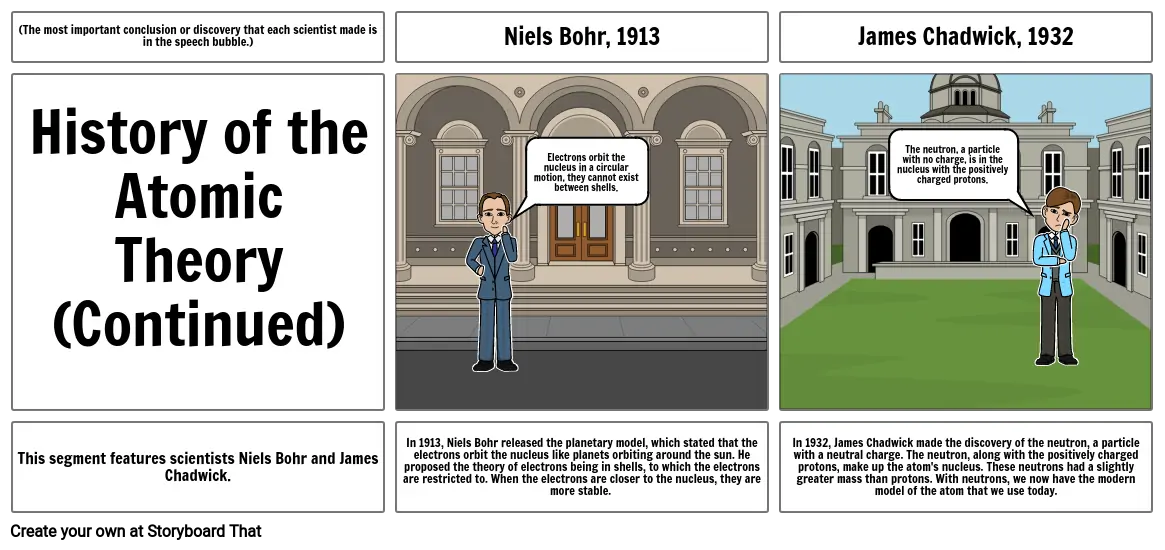
Niels Bohr, 1913
James Chadwick, 1932
This segment features scientists Niels Bohr and James Chadwick.
In 1913, Niels Bohr released the planetary model, which stated that the electrons orbit the nucleus like planets orbiting around the sun. He proposed the theory of electrons being in shells, to which the electrons are restricted to. When the electrons are closer to the nucleus, they are more stable.
In 1932, James Chadwick made the discovery of the neutron, a particle with a neutral charge. The neutron, along with the positively charged protons, make up the atom's nucleus. These neutrons had a slightly greater mass than protons. With neutrons, we now have the modern model of the atom that we use today.
History of the Atomic Theory (Continued)
Electrons orbit the nucleus in a circular motion, they cannot exist between shells.
The neutron, a particle with no charge, is in the nucleus with the positively charged protons.
(The most important conclusion or discovery that each scientist made is in the speech bubble.)
Niels Bohr, 1913
James Chadwick, 1932
This segment features scientists Niels Bohr and James Chadwick.
In 1913, Niels Bohr released the planetary model, which stated that the electrons orbit the nucleus like planets orbiting around the sun. He proposed the theory of electrons being in shells, to which the electrons are restricted to. When the electrons are closer to the nucleus, they are more stable.
In 1932, James Chadwick made the discovery of the neutron, a particle with a neutral charge. The neutron, along with the positively charged protons, make up the atom's nucleus. These neutrons had a slightly greater mass than protons. With neutrons, we now have the modern model of the atom that we use today.
History of the Atomic Theory (Continued)
Electrons orbit the nucleus in a circular motion, they cannot exist between shells.
The neutron, a particle with no charge, is in the nucleus with the positively charged protons.
(The most important conclusion or discovery that each scientist made is in the speech bubble.)
Niels Bohr, 1913
James Chadwick, 1932
This segment features scientists Niels Bohr and James Chadwick.
In 1913, Niels Bohr released the planetary model, which stated that the electrons orbit the nucleus like planets orbiting around the sun. He proposed the theory of electrons being in shells, to which the electrons are restricted to. When the electrons are closer to the nucleus, they are more stable.
In 1932, James Chadwick made the discovery of the neutron, a particle with a neutral charge. The neutron, along with the positively charged protons, make up the atom's nucleus. These neutrons had a slightly greater mass than protons. With neutrons, we now have the modern model of the atom that we use today.
History of the Atomic Theory (Continued)
Electrons orbit the nucleus in a circular motion, they cannot exist between shells.
The neutron, a particle with no charge, is in the nucleus with the positively charged protons.
(The most important conclusion or discovery that each scientist made is in the speech bubble.)
Niels Bohr, 1913
James Chadwick, 1932
This segment features scientists Niels Bohr and James Chadwick.
In 1913, Niels Bohr released the planetary model, which stated that the electrons orbit the nucleus like planets orbiting around the sun. He proposed the theory of electrons being in shells, to which the electrons are restricted to. When the electrons are closer to the nucleus, they are more stable.
In 1932, James Chadwick made the discovery of the neutron, a particle with a neutral charge. The neutron, along with the positively charged protons, make up the atom's nucleus. These neutrons had a slightly greater mass than protons. With neutrons, we now have the modern model of the atom that we use today.
History of the Atomic Theory (Continued)
Electrons orbit the nucleus in a circular motion, they cannot exist between shells.
The neutron, a particle with no charge, is in the nucleus with the positively charged protons.
Storyboard Beskrivning
Storyboard Text
- (The most important conclusion or discovery that each scientist made is in the speech bubble.)
- History of the Atomic Theory (Continued)
- Niels Bohr, 1913
- Electrons orbit the nucleus in a circular motion, they cannot exist between shells.
- James Chadwick, 1932
- The neutron, a particle with no charge, is in the nucleus with the positively charged protons.
- This segment features scientists Niels Bohr and James Chadwick.
- In 1913, Niels Bohr released the planetary model, which stated that the electrons orbit the nucleus like planets orbiting around the sun. He proposed the theory of electrons being in shells, to which the electrons are restricted to. When the electrons are closer to the nucleus, they are more stable.
- In 1932, James Chadwick made the discovery of the neutron, a particle with a neutral charge. The neutron, along with the positively charged protons, make up the atom's nucleus. These neutrons had a slightly greater mass than protons. With neutrons, we now have the modern model of the atom that we use today.


