ATOMIC THEORY
Storyboard Text
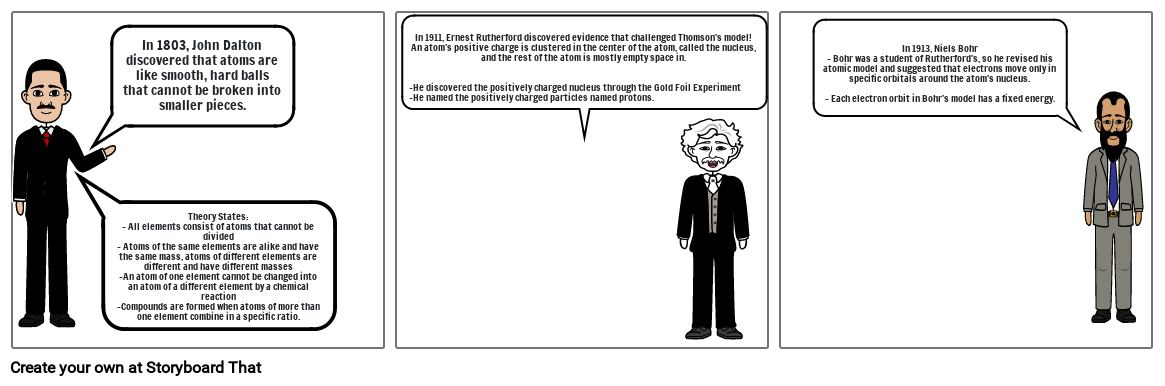
- Theory States:- All elements consist of atoms that cannot be divided - Atoms of the same elements are alike and have the same mass, atoms of different elements are different and have different masses-An atom of one element cannot be changed into an atom of a different element by a chemical reaction -Compounds are formed when atoms of more than one element combine in a specific ratio.
- In 1803, John Dalton discovered that atoms are like smooth, hard balls that cannot be broken into smaller pieces.
- In 1911, Ernest Rutherford discovered evidence that challenged Thomson's model!An atom’s positive charge is clustered in the center of the atom, called the nucleus, and the rest of the atom is mostly empty space in.-He discovered the positively charged nucleus through the Gold Foil Experiment-He named the positively charged particles named protons.
- In 1913, Niels Bohr- Bohr was a student of Rutherford's, so he revised his atomic model and suggested that electrons move only in specific orbitals around the atom's nucleus.- Each electron orbit in Bohr’s model has a fixed energy.
Över 30 miljoner storyboards skapade

