Atomic structure subject enrichment
Storyboard Text
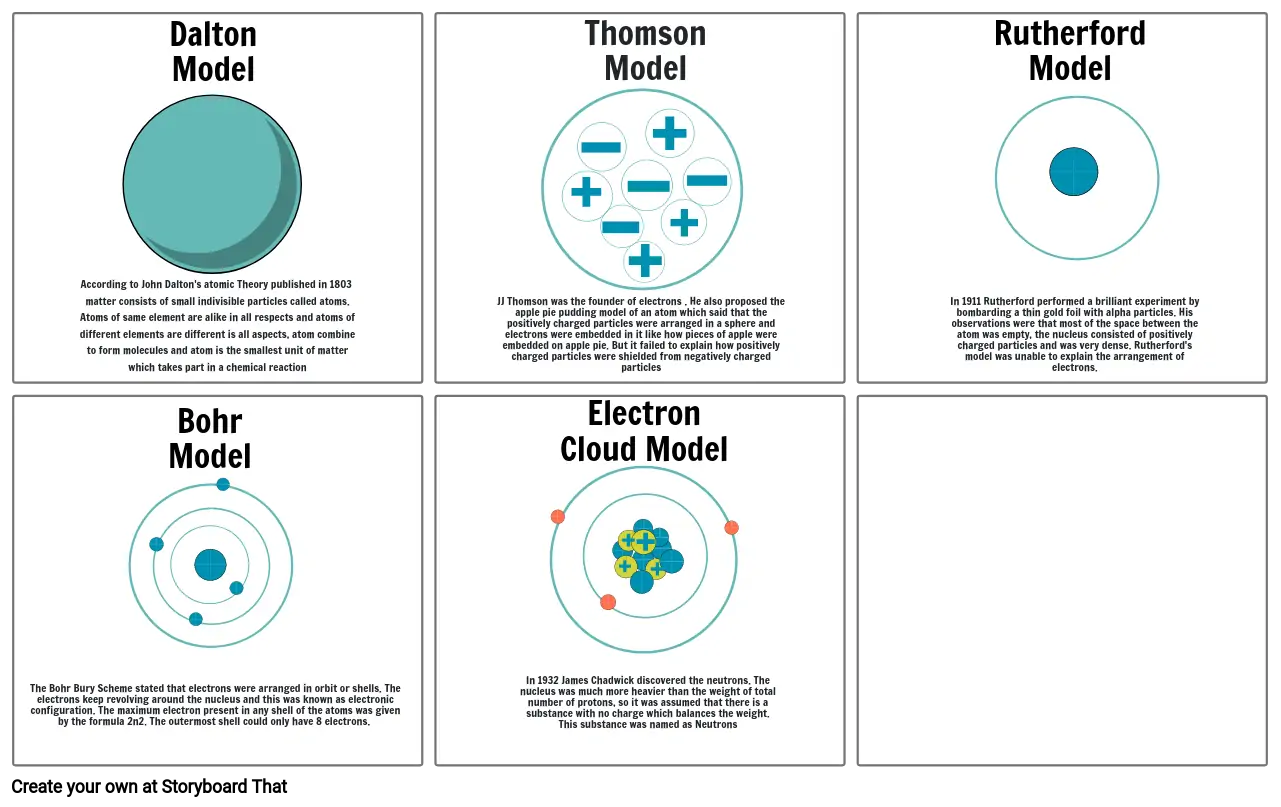
- According to John Dalton's atomic Theory published in 1803 matter consists of small indivisible particles called atoms. Atoms of same element are alike in all respects and atoms of different elements are different is all aspects, atom combine to form molecules and atom is the smallest unit of matter which takes part in a chemical reaction
- Dalton Model
- JJ Thomson was the founder of electrons . He also proposed the apple pie pudding model of an atom which said that the positively charged particles were arranged in a sphere and electrons were embedded in it like how pieces of apple were embedded on apple pie. But it failed to explain how positively charged particles were shielded from negatively charged particles
- Electron Cloud Model
- Thomson Model
- In 1911 Rutherford performed a brilliant experiment by bombarding a thin gold foil with alpha particles. His observations were that most of the space between the atom was empty, the nucleus consisted of positively charged particles and was very dense. Rutherford's model was unable to explain the arrangement of electrons.
- Rutherford Model
- The Bohr Bury Scheme stated that electrons were arranged in orbit or shells. The electrons keep revolving around the nucleus and this was known as electronic configuration. The maximum electron present in any shell of the atoms was given by the formula 2n2. The outermost shell could only have 8 electrons.
- Bohr Model
- In 1932 James Chadwick discovered the neutrons. The nucleus was much more heavier than the weight of total number of protons, so it was assumed that there is a substance with no charge which balances the weight. This substance was named as Neutrons
Over 30 Million Storyboards Created

