atoms
Storyboard Text
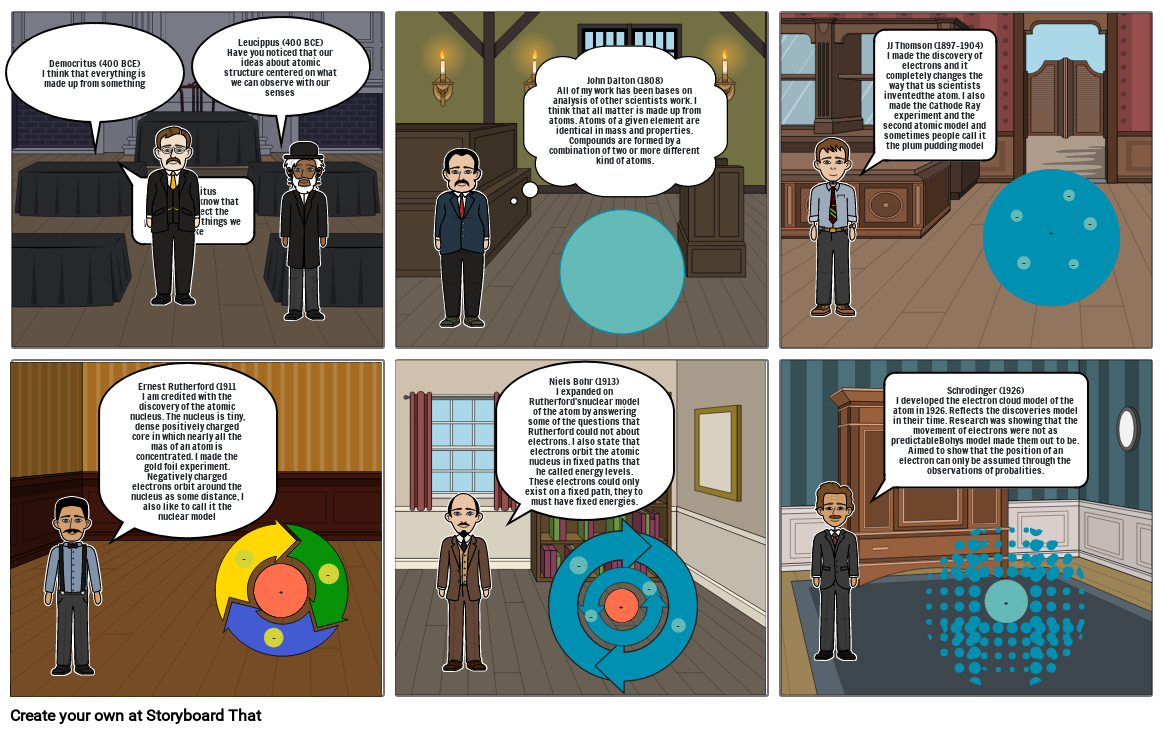
- Democritus (400 BCE)I think that everything is made up from something
- DemocritusDid you also know that atoms reflect the properties of things we make
- Leucippus (400 BCE)Have you noticed that our ideas about atomic structure centered on what we can observe with our senses
- John Dalton (1808)All of my work has been bases on analysis of other scientists work. I think that all matter is made up from atoms. Atoms of a given element are identical in mass and properties. Compounds are formed by a combination of two or more different kind of atoms.
- JJ Thomson (1897-1904)I made the discovery of electrons and it completely changes the way that us scientists inventedthe atom. I also made the Cathode Ray experiment and the second atomic model and sometimes people call it the plum pudding model
- -
- -
- +
- -
- -
- -
- Ernest Rutherford (1911I am credited with the discovery of the atomic nucleus. The nucleus is tiny, dense positively charged core in which nearly all the mas of an atom is concentrated. I made the gold foil experiment. Negatively charged electrons orbit around the nucleus as some distance, I also like to call it the nuclear model
-
- -
- +
- -
- -
- Niels Bohr (1913)I expanded on Rutherford'snuclear model of the atom by answering some of the questions that Rutherford could not about electrons. I also state that electrons orbit the atomic nucleus in fixed paths that he called energy levels. These electrons could only exist on a fixed path, they to must have fixed energies.
- -
- -
- +
- -
- -
- Schrodinger (1926)I developed the electron cloud model of the atom in 1926. Reflects the discoveries model in their time. Research was showing that the movement of electrons were not as predictableBohys model made them out to be. Aimed to show that the position of an electron can only be assumed through the observations of probalities.
-
-
- +
Over 30 Million Storyboards Created

