Chemistry
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
This seems interesting.
But I only know the first step. Hope somebody finishes what I started
Magnesium + Nitrogen gas
Magnesium Nitride
Step 1: Convert the given problem into a word equation
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
Ohhh! An unfinished chemical equation. I'm gonna test my inner scientist abilities
******
Magnesium + Nitrogen gas
Magnesium Nitride
Step 2: Convert the word equation into a chemical equation.
Note: Lookout for diatomic substances like nitrogen
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
Am I obliged to continue this? Arghhh why am I in STEM!
****************
Magnesium + Nitrogen gas
Magnesium Nitride
Step 3: Balance the chemical equation by adding coefficients on both sides so that both sides have the same number of atoms.This satisfy the law of conservation of matter
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
This seems interesting.
But I only know the first step. Hope somebody finishes what I started
Magnesium + Nitrogen gas
Magnesium Nitride
Step 1: Convert the given problem into a word equation
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
Ohhh! An unfinished chemical equation. I'm gonna test my inner scientist abilities
******
Magnesium + Nitrogen gas
Magnesium Nitride
Step 2: Convert the word equation into a chemical equation.
Note: Lookout for diatomic substances like nitrogen
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
Am I obliged to continue this? Arghhh why am I in STEM!
****************
Magnesium + Nitrogen gas
Magnesium Nitride
Step 3: Balance the chemical equation by adding coefficients on both sides so that both sides have the same number of atoms.This satisfy the law of conservation of matter
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
This seems interesting.
But I only know the first step. Hope somebody finishes what I started
Magnesium + Nitrogen gas
Magnesium Nitride
Step 1: Convert the given problem into a word equation
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
Ohhh! An unfinished chemical equation. I'm gonna test my inner scientist abilities
******
Magnesium + Nitrogen gas
Magnesium Nitride
Step 2: Convert the word equation into a chemical equation.
Note: Lookout for diatomic substances like nitrogen
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
Am I obliged to continue this? Arghhh why am I in STEM!
****************
Magnesium + Nitrogen gas
Magnesium Nitride
Step 3: Balance the chemical equation by adding coefficients on both sides so that both sides have the same number of atoms.This satisfy the law of conservation of matter
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
This seems interesting.
But I only know the first step. Hope somebody finishes what I started
Magnesium + Nitrogen gas
Magnesium Nitride
Step 1: Convert the given problem into a word equation
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
Ohhh! An unfinished chemical equation. I'm gonna test my inner scientist abilities
******
Magnesium + Nitrogen gas
Magnesium Nitride
Step 2: Convert the word equation into a chemical equation.
Note: Lookout for diatomic substances like nitrogen
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
Am I obliged to continue this? Arghhh why am I in STEM!
****************
Magnesium + Nitrogen gas
Magnesium Nitride
Step 3: Balance the chemical equation by adding coefficients on both sides so that both sides have the same number of atoms.This satisfy the law of conservation of matter
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
This seems interesting.
But I only know the first step. Hope somebody finishes what I started
Magnesium + Nitrogen gas
Magnesium Nitride
Step 1: Convert the given problem into a word equation
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
Ohhh! An unfinished chemical equation. I'm gonna test my inner scientist abilities
******
Magnesium + Nitrogen gas
Magnesium Nitride
Step 2: Convert the word equation into a chemical equation.
Note: Lookout for diatomic substances like nitrogen
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
Am I obliged to continue this? Arghhh why am I in STEM!
****************
Magnesium + Nitrogen gas
Magnesium Nitride
Step 3: Balance the chemical equation by adding coefficients on both sides so that both sides have the same number of atoms.This satisfy the law of conservation of matter
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
This seems interesting.
But I only know the first step. Hope somebody finishes what I started
Magnesium + Nitrogen gas
Magnesium Nitride
Step 1: Convert the given problem into a word equation
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
Ohhh! An unfinished chemical equation. I'm gonna test my inner scientist abilities
******
Magnesium + Nitrogen gas
Magnesium Nitride
Step 2: Convert the word equation into a chemical equation.
Note: Lookout for diatomic substances like nitrogen
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
Am I obliged to continue this? Arghhh why am I in STEM!
****************
Magnesium + Nitrogen gas
Magnesium Nitride
Step 3: Balance the chemical equation by adding coefficients on both sides so that both sides have the same number of atoms.This satisfy the law of conservation of matter
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
This seems interesting.
But I only know the first step. Hope somebody finishes what I started
Magnesium + Nitrogen gas
Magnesium Nitride
Step 1: Convert the given problem into a word equation
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
Ohhh! An unfinished chemical equation. I'm gonna test my inner scientist abilities
******
Magnesium + Nitrogen gas
Magnesium Nitride
Step 2: Convert the word equation into a chemical equation.
Note: Lookout for diatomic substances like nitrogen
Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
Am I obliged to continue this? Arghhh why am I in STEM!
****************
Magnesium + Nitrogen gas
Magnesium Nitride
Step 3: Balance the chemical equation by adding coefficients on both sides so that both sides have the same number of atoms.This satisfy the law of conservation of matter
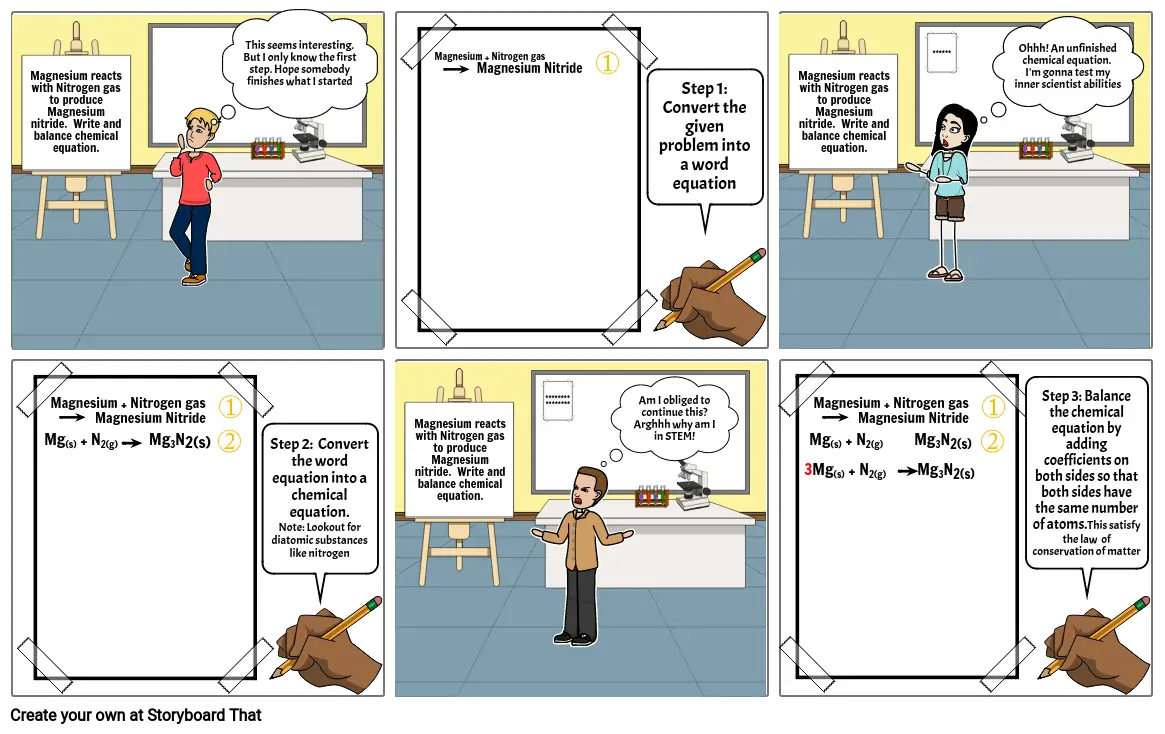
Storyboard Text
- Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
- This seems interesting. But I only know the first step. Hope somebody finishes what I started
- Magnesium + Nitrogen gas Magnesium Nitride
- Step 1: Convert the given problem into a word equation
- Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
- ******
- Ohhh! An unfinished chemical equation. I'm gonna test my inner scientist abilities
- Mg(s) + N2(g) Mg3N2(s)
- Magnesium + Nitrogen gas Magnesium Nitride
- Step 2: Convert the word equation into a chemical equation. Note: Lookout for diatomic substances like nitrogen
- Magnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance chemical equation.
- ****************
- Am I obliged to continue this? Arghhh why am I in STEM!
- 3Mg(s) + N2(g) Mg3N2(s)
- Mg(s) + N2(g) Mg3N2(s)
- Magnesium + Nitrogen gas Magnesium Nitride
- Step 3: Balance the chemical equation by adding coefficients on both sides so that both sides have the same number of atoms.This satisfy the law of conservation of matter



