Ice cube melting skit
Storyboard Text
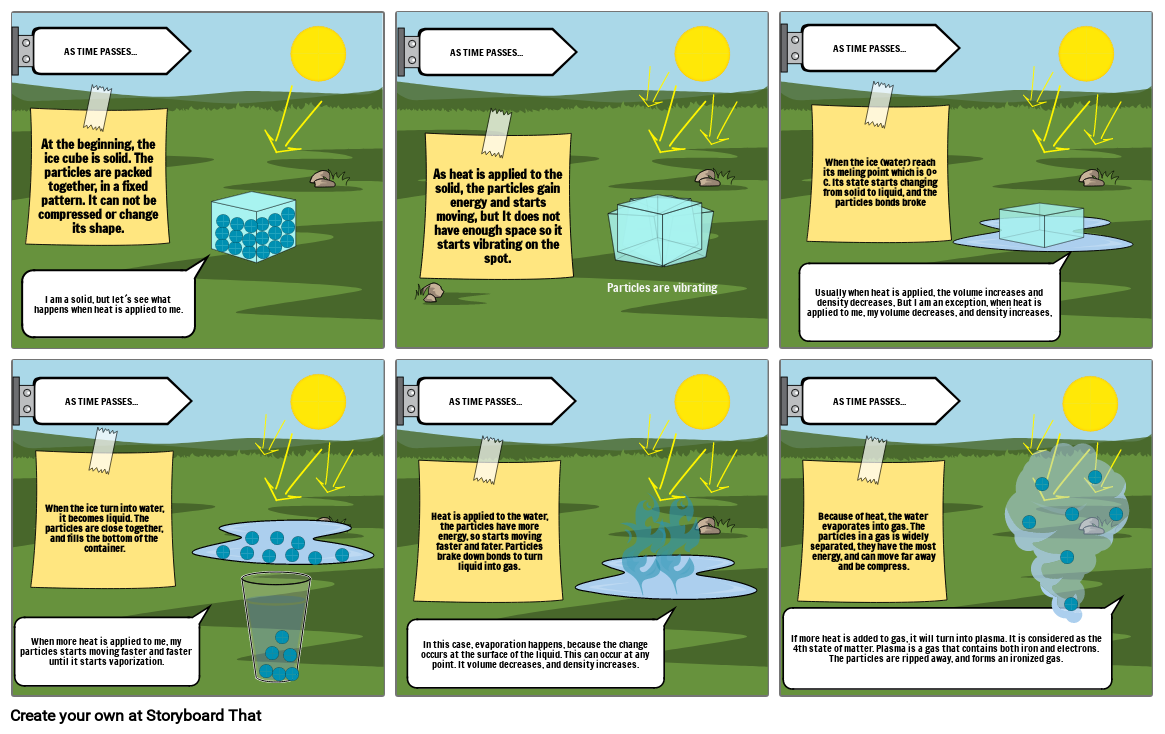
- AS TIME PASSES...
- I am a solid, but let´s see what happens when heat is applied to me.
- At the beginning, the ice cube is solid. The particles are packed together, in a fixed pattern. It can not be compressed or change its shape.
- AS TIME PASSES...
- As heat is applied to the solid, the particles gain energy and starts moving, but It does not have enough space so it starts vibrating on the spot.
- Particles are vibrating
- AS TIME PASSES...
- Usually when heat is applied, the volume increases and density decreases, But I am an exception, when heat is applied to me, my volume decreases, and density increases,
- When the ice (water) reach its meling point which is 0º C. Its state starts changing from solid to liquid, and the particles bonds broke
- AS TIME PASSES...
- When more heat is applied to me, my particles starts moving faster and faster until it starts vaporization.
- When the ice turn into water, it becomes liquid. The particles are close together, and fills the bottom of the container.
- AS TIME PASSES...
- In this case, evaporation happens, because the change occurs at the surface of the liquid. This can occur at any point. It volume decreases, and density increases.
- Heat is applied to the water, the particles have more energy, so starts moving faster and fater. Particles brake down bonds to turn liquid into gas.
- AS TIME PASSES...
- If more heat is added to gas, it will turn into plasma. It is considered as the 4th state of matter. Plasma is a gas that contains both iron and electrons. The particles are ripped away, and forms an ironized gas.
- Because of heat, the water evaporates into gas. The particles in a gas is widely separated, they have the most energy, and can move far away and be compress.
Over 30 Million Storyboards Created

