Unknown Story
Storyboard Text
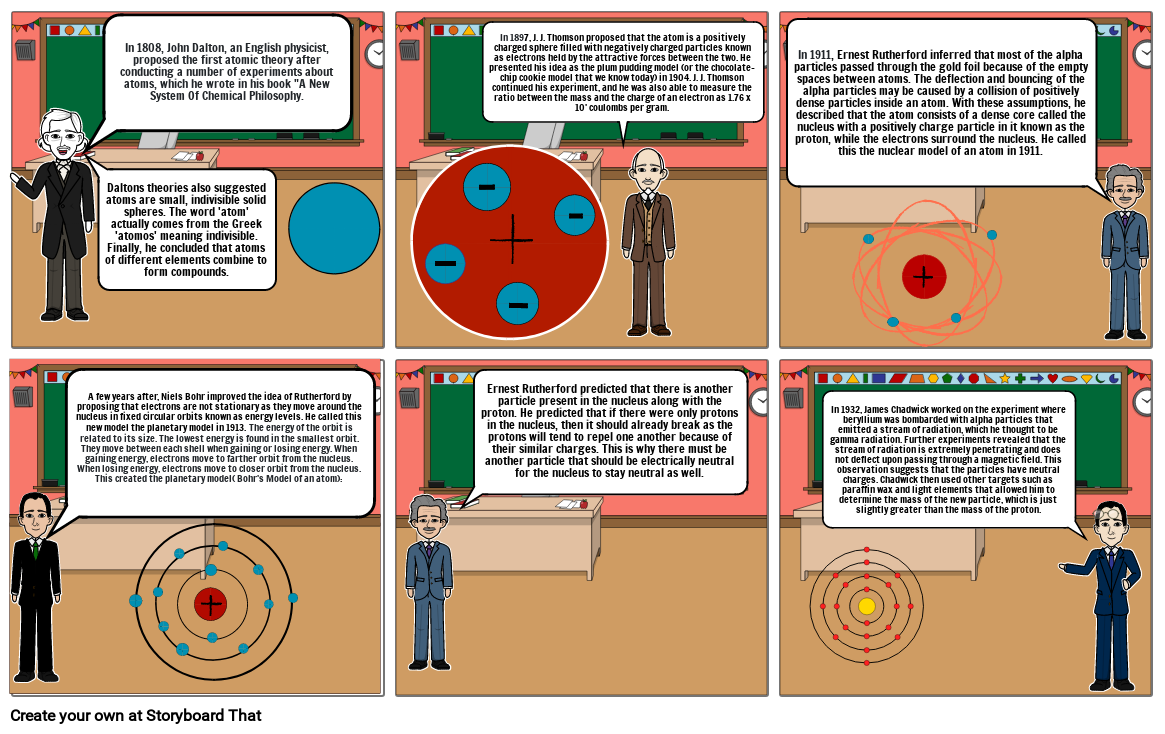
- In 1808, John Dalton, an English physicist, proposed the first atomic theory after conducting a number of experiments about atoms, which he wrote in his book "A New System Of Chemical Philosophy.
- Daltons theories also suggested atoms are small, indivisible solid spheres. The word 'atom' actually comes from the Greek 'atomos' meaning indivisible. Finally, he concluded that atoms of different elements combine to form compounds.
- In 1897, J. J. Thomson proposed that the atom is a positively charged sphere filled with negatively charged particles known as electrons held by the attractive forces between the two. He presented his idea as the plum pudding model (or the chocolate-chip cookie model that we know today) in 1904. J. J. Thomson continued his experiment, and he was also able to measure the ratio between the mass and the charge of an electron as 1.76 x 10' coulombs per gram.
- In 1911, Ernest Rutherford inferred that most of the alpha particles passed through the gold foil because of the empty spaces between atoms. The deflection and bouncing of the alpha particles may be caused by a collision of positively dense particles inside an atom. With these assumptions, he described that the atom consists of a dense core called the nucleus with a positively charge particle in it known as the proton, while the electrons surround the nucleus. He called this the nuclear model of an atom in 1911.
- A few years after, Niels Bohr improved the idea of Rutherford by proposing that electrons are not stationary as they move around the nucleus in fixed circular orbits known as energy levels. He called this new model the planetary model in 1913. The energy of the orbit is related to its size. The lowest energy is found in the smallest orbit. They move between each shell when gaining or losing energy. When gaining energy, electrons move to farther orbit from the nucleus. When losing energy, electrons move to closer orbit from the nucleus. This created the planetary model( Bohr's Model of an atom):
- Ernest Rutherford predicted that there is another particle present in the nucleus along with the proton. He predicted that if there were only protons in the nucleus, then it should already break as the protons will tend to repel one another because of their similar charges. This is why there must be another particle that should be electrically neutral for the nucleus to stay neutral as well.
- In 1932, James Chadwick worked on the experiment where beryllium was bombarded with alpha particles that emitted a stream of radiation, which he thought to be gamma radiation. Further experiments revealed that the stream of radiation is extremely penetrating and does not deflect upon passing through a magnetic field. This observation suggests that the particles have neutral charges. Chadwick then used other targets such as paraffin wax and light elements that allowed him to determine the mass of the new particle, which is just slightly greater than the mass of the proton.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
