charles law
Volume – Temperature or Charle’s Law is an experimental gas law that describes how gases tend to expand when heated. The law states that the volume of a given quantity of gas is directly proportional to the kelvin temperature, at constant pressure. The law simply means that as temperature increases, the volume is also increases. Any change in Kelvin temperature would likewise change the volume of the gas
Who formulated this law?
The law was named after scientist Jacques
What is the mathematical expression of Charles Law?
The mathematical expression of Chales Law goes :
V α T(kelvin) at constant pressure
V/T =K (a constant) so therefore V1/T =K
Final equations is V1/T1 = V2 /T2
Very good!
Whereas, V1 is the initial volume and V2 is the final volumeT1 is the initial temperature and T2 is the final temperature. Take note that temperature should be in Kelvin,
so what is the formula of Kelvin?
Let apply Charles Law by solving this problem.
1. Kr gas occupies a volume of 1.75 L at 25.0 C. Determine the volume of the gas if the temperature is increased to 50.0 C. What are the given?
To convert Celsius to Kelvin, the formula is K= C + 273.15
Given:
V1=1.75 L, T1= 25.0 C, T2= 50.0 C, V2=?
Solution:
Convert C to K
T1 = 25.0 C + 273.15 = 298.15 K
T2 = 50.0 C + 273.15 = 323.15
Formula: V1/T1 = V2 /T2
Substitution and cross multiply
=1.75 L = V2
298.15 K 323.15 K
final answer =1.90 L
Volume – Temperature or Charle’s Law is an experimental gas law that describes how gases tend to expand when heated. The law states that the volume of a given quantity of gas is directly proportional to the kelvin temperature, at constant pressure. The law simply means that as temperature increases, the volume is also increases. Any change in Kelvin temperature would likewise change the volume of the gas
Who formulated this law?
The law was named after scientist Jacques
What is the mathematical expression of Charles Law?
The mathematical expression of Chales Law goes :
V α T(kelvin) at constant pressure
V/T =K (a constant) so therefore V1/T =K
Final equations is V1/T1 = V2 /T2
Very good!
Whereas, V1 is the initial volume and V2 is the final volumeT1 is the initial temperature and T2 is the final temperature. Take note that temperature should be in Kelvin,
so what is the formula of Kelvin?
Let apply Charles Law by solving this problem.
1. Kr gas occupies a volume of 1.75 L at 25.0 C. Determine the volume of the gas if the temperature is increased to 50.0 C. What are the given?
To convert Celsius to Kelvin, the formula is K= C + 273.15
Given:
V1=1.75 L, T1= 25.0 C, T2= 50.0 C, V2=?
Solution:
Convert C to K
T1 = 25.0 C + 273.15 = 298.15 K
T2 = 50.0 C + 273.15 = 323.15
Formula: V1/T1 = V2 /T2
Substitution and cross multiply
=1.75 L = V2
298.15 K 323.15 K
final answer =1.90 L
Volume – Temperature or Charle’s Law is an experimental gas law that describes how gases tend to expand when heated. The law states that the volume of a given quantity of gas is directly proportional to the kelvin temperature, at constant pressure. The law simply means that as temperature increases, the volume is also increases. Any change in Kelvin temperature would likewise change the volume of the gas
Who formulated this law?
The law was named after scientist Jacques
What is the mathematical expression of Charles Law?
The mathematical expression of Chales Law goes :
V α T(kelvin) at constant pressure
V/T =K (a constant) so therefore V1/T =K
Final equations is V1/T1 = V2 /T2
Very good!
Whereas, V1 is the initial volume and V2 is the final volumeT1 is the initial temperature and T2 is the final temperature. Take note that temperature should be in Kelvin,
so what is the formula of Kelvin?
Let apply Charles Law by solving this problem.
1. Kr gas occupies a volume of 1.75 L at 25.0 C. Determine the volume of the gas if the temperature is increased to 50.0 C. What are the given?
To convert Celsius to Kelvin, the formula is K= C + 273.15
Given:
V1=1.75 L, T1= 25.0 C, T2= 50.0 C, V2=?
Solution:
Convert C to K
T1 = 25.0 C + 273.15 = 298.15 K
T2 = 50.0 C + 273.15 = 323.15
Formula: V1/T1 = V2 /T2
Substitution and cross multiply
=1.75 L = V2
298.15 K 323.15 K
final answer =1.90 L
Volume – Temperature or Charle’s Law is an experimental gas law that describes how gases tend to expand when heated. The law states that the volume of a given quantity of gas is directly proportional to the kelvin temperature, at constant pressure. The law simply means that as temperature increases, the volume is also increases. Any change in Kelvin temperature would likewise change the volume of the gas
Who formulated this law?
The law was named after scientist Jacques
What is the mathematical expression of Charles Law?
The mathematical expression of Chales Law goes :
V α T(kelvin) at constant pressure
V/T =K (a constant) so therefore V1/T =K
Final equations is V1/T1 = V2 /T2
Very good!
Whereas, V1 is the initial volume and V2 is the final volumeT1 is the initial temperature and T2 is the final temperature. Take note that temperature should be in Kelvin,
so what is the formula of Kelvin?
Let apply Charles Law by solving this problem.
1. Kr gas occupies a volume of 1.75 L at 25.0 C. Determine the volume of the gas if the temperature is increased to 50.0 C. What are the given?
To convert Celsius to Kelvin, the formula is K= C + 273.15
Given:
V1=1.75 L, T1= 25.0 C, T2= 50.0 C, V2=?
Solution:
Convert C to K
T1 = 25.0 C + 273.15 = 298.15 K
T2 = 50.0 C + 273.15 = 323.15
Formula: V1/T1 = V2 /T2
Substitution and cross multiply
=1.75 L = V2
298.15 K 323.15 K
final answer =1.90 L
Volume – Temperature or Charle’s Law is an experimental gas law that describes how gases tend to expand when heated. The law states that the volume of a given quantity of gas is directly proportional to the kelvin temperature, at constant pressure. The law simply means that as temperature increases, the volume is also increases. Any change in Kelvin temperature would likewise change the volume of the gas
Who formulated this law?
The law was named after scientist Jacques
What is the mathematical expression of Charles Law?
The mathematical expression of Chales Law goes :
V α T(kelvin) at constant pressure
V/T =K (a constant) so therefore V1/T =K
Final equations is V1/T1 = V2 /T2
Very good!
Whereas, V1 is the initial volume and V2 is the final volumeT1 is the initial temperature and T2 is the final temperature. Take note that temperature should be in Kelvin,
so what is the formula of Kelvin?
Let apply Charles Law by solving this problem.
1. Kr gas occupies a volume of 1.75 L at 25.0 C. Determine the volume of the gas if the temperature is increased to 50.0 C. What are the given?
To convert Celsius to Kelvin, the formula is K= C + 273.15
Given:
V1=1.75 L, T1= 25.0 C, T2= 50.0 C, V2=?
Solution:
Convert C to K
T1 = 25.0 C + 273.15 = 298.15 K
T2 = 50.0 C + 273.15 = 323.15
Formula: V1/T1 = V2 /T2
Substitution and cross multiply
=1.75 L = V2
298.15 K 323.15 K
final answer =1.90 L
Volume – Temperature or Charle’s Law is an experimental gas law that describes how gases tend to expand when heated. The law states that the volume of a given quantity of gas is directly proportional to the kelvin temperature, at constant pressure. The law simply means that as temperature increases, the volume is also increases. Any change in Kelvin temperature would likewise change the volume of the gas
Who formulated this law?
The law was named after scientist Jacques
What is the mathematical expression of Charles Law?
The mathematical expression of Chales Law goes :
V α T(kelvin) at constant pressure
V/T =K (a constant) so therefore V1/T =K
Final equations is V1/T1 = V2 /T2
Very good!
Whereas, V1 is the initial volume and V2 is the final volumeT1 is the initial temperature and T2 is the final temperature. Take note that temperature should be in Kelvin,
so what is the formula of Kelvin?
Let apply Charles Law by solving this problem.
1. Kr gas occupies a volume of 1.75 L at 25.0 C. Determine the volume of the gas if the temperature is increased to 50.0 C. What are the given?
To convert Celsius to Kelvin, the formula is K= C + 273.15
Given:
V1=1.75 L, T1= 25.0 C, T2= 50.0 C, V2=?
Solution:
Convert C to K
T1 = 25.0 C + 273.15 = 298.15 K
T2 = 50.0 C + 273.15 = 323.15
Formula: V1/T1 = V2 /T2
Substitution and cross multiply
=1.75 L = V2
298.15 K 323.15 K
final answer =1.90 L
Volume – Temperature or Charle’s Law is an experimental gas law that describes how gases tend to expand when heated. The law states that the volume of a given quantity of gas is directly proportional to the kelvin temperature, at constant pressure. The law simply means that as temperature increases, the volume is also increases. Any change in Kelvin temperature would likewise change the volume of the gas
Who formulated this law?
The law was named after scientist Jacques
What is the mathematical expression of Charles Law?
The mathematical expression of Chales Law goes :
V α T(kelvin) at constant pressure
V/T =K (a constant) so therefore V1/T =K
Final equations is V1/T1 = V2 /T2
Very good!
Whereas, V1 is the initial volume and V2 is the final volumeT1 is the initial temperature and T2 is the final temperature. Take note that temperature should be in Kelvin,
so what is the formula of Kelvin?
Let apply Charles Law by solving this problem.
1. Kr gas occupies a volume of 1.75 L at 25.0 C. Determine the volume of the gas if the temperature is increased to 50.0 C. What are the given?
To convert Celsius to Kelvin, the formula is K= C + 273.15
Given:
V1=1.75 L, T1= 25.0 C, T2= 50.0 C, V2=?
Solution:
Convert C to K
T1 = 25.0 C + 273.15 = 298.15 K
T2 = 50.0 C + 273.15 = 323.15
Formula: V1/T1 = V2 /T2
Substitution and cross multiply
=1.75 L = V2
298.15 K 323.15 K
final answer =1.90 L
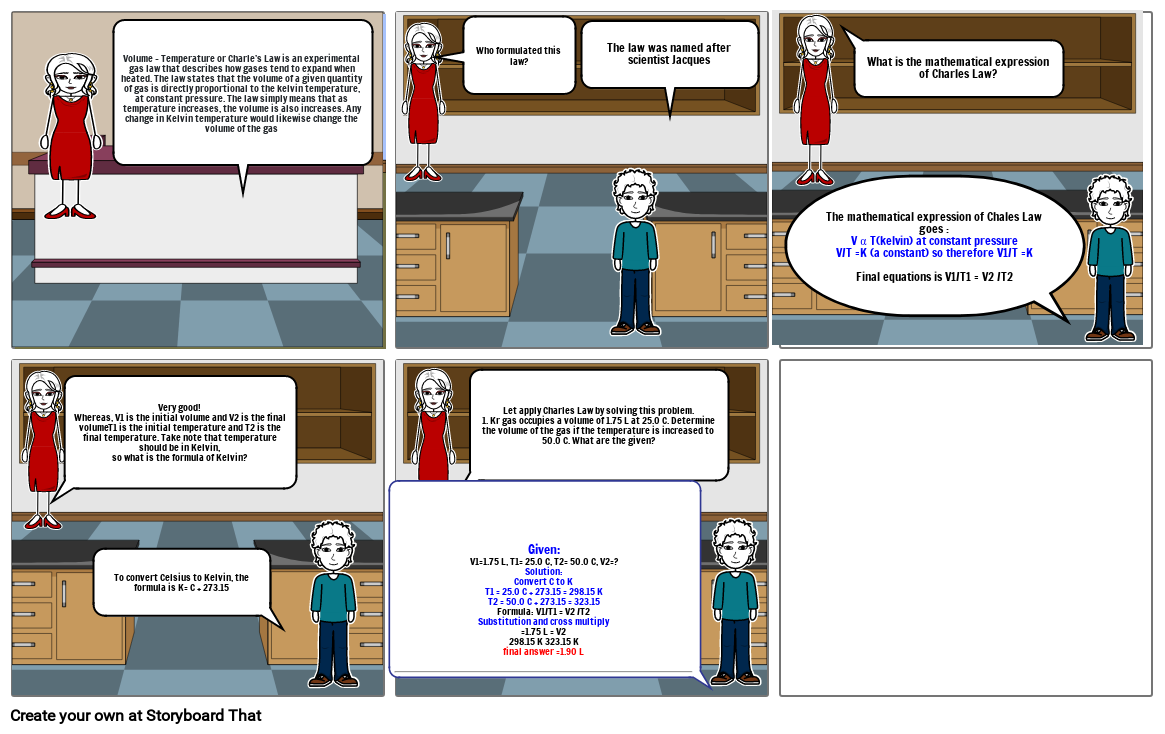
Storyboard Text
- Volume – Temperature or Charle’s Law is an experimental gas law that describes how gases tend to expand when heated. The law states that the volume of a given quantity of gas is directly proportional to the kelvin temperature, at constant pressure. The law simply means that as temperature increases, the volume is also increases. Any change in Kelvin temperature would likewise change the volume of the gas
- Who formulated this law?
- The law was named after scientist Jacques
- The mathematical expression of Chales Law goes :V α T(kelvin) at constant pressureV/T =K (a constant) so therefore V1/T =KFinal equations is V1/T1 = V2 /T2
- What is the mathematical expression of Charles Law?
- Very good! Whereas, V1 is the initial volume and V2 is the final volumeT1 is the initial temperature and T2 is the final temperature. Take note that temperature should be in Kelvin,so what is the formula of Kelvin?
- To convert Celsius to Kelvin, the formula is K= C + 273.15
- Given: V1=1.75 L, T1= 25.0 C, T2= 50.0 C, V2=?Solution:Convert C to KT1 = 25.0 C + 273.15 = 298.15 KT2 = 50.0 C + 273.15 = 323.15Formula: V1/T1 = V2 /T2Substitution and cross multiply=1.75 L = V2 298.15 K 323.15 Kfinal answer =1.90 L
- Let apply Charles Law by solving this problem.1. Kr gas occupies a volume of 1.75 L at 25.0 C. Determine the volume of the gas if the temperature is increased to 50.0 C. What are the given?



