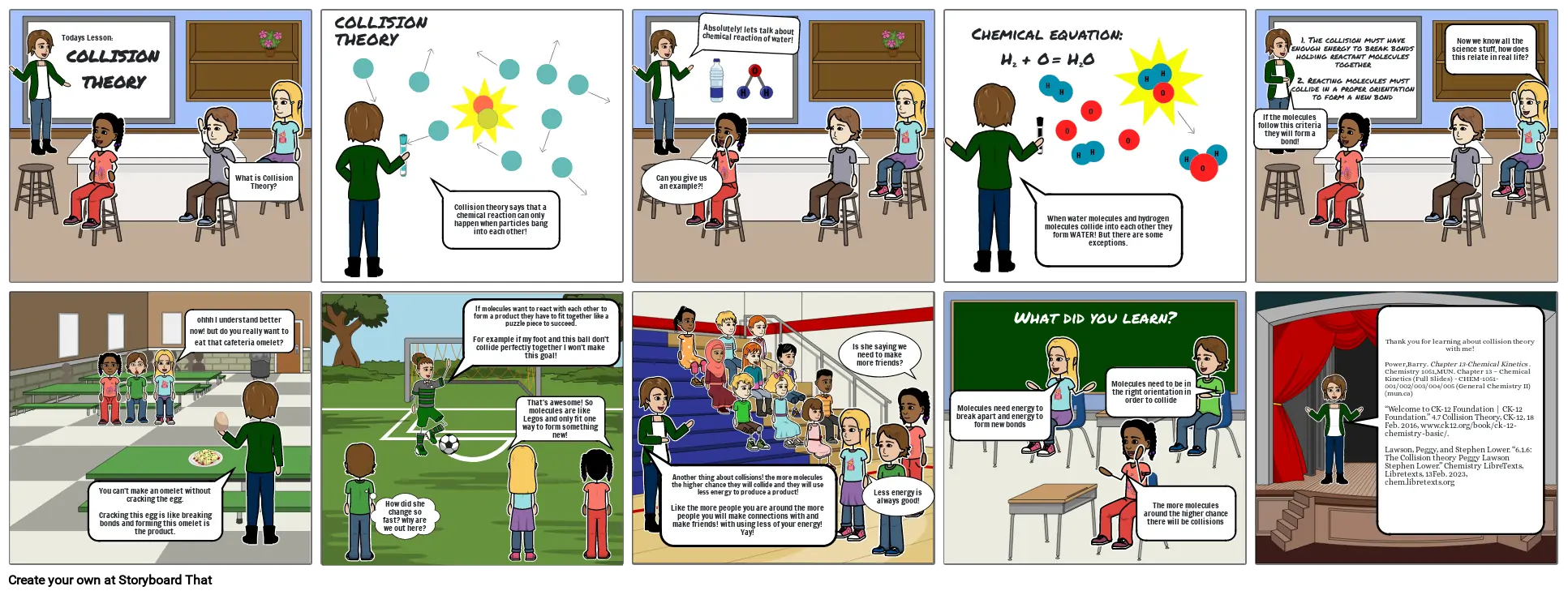
What is collision theory
Storyboard Text
- COLLISION THEORY
- Todays Lesson:
- What is Collision Theory?
- COLLISION THEORY
- Collision theory says that a chemical reaction can only happen when particles bang into each other!
- Can you give us an example?!
- Absolutely! lets talk about chemical reaction of water!
- Chemical equation: H2 + O = H2O
- When water molecules and hydrogen molecules collide into each other they form WATER! But there are some exceptions.
- H
- H
- O
- H
- O
- H
- O
- H
- O
- H
- H
- O
- H
- If the molecules follow this criteria they will form a bond!
- 1. The collision must have enough energy to break bonds holding reactant molecules together2. Reacting molecules must collide in a proper orientation to form a new bond
- Now we know all the science stuff, how does this relate in real life?
- You can't make an omelet without cracking the egg.Cracking this egg is like breaking bonds and forming this omelet is the product.
- ohhh I understand better now! but do you really want to eat that cafeteria omelet?
- How did she change so fast? why are we out here?
- If molecules want to react with each other to form a product they have to fit together like a puzzle piece to succeed.For example if my foot and this ball don't collide perfectly together I won't make this goal!
- That's awesome! So molecules are like Legos and only fit one way to form something new!
- Another thing about collisions! the more molecules the higher chance they will collide and they will use less energy to produce a product!Like the more people you are around the more people you will make connections with and make friends! with using less of your energy! Yay!
- Is she saying we need to make more friends?
- Less energy is always good!
- Molecules need energy to break apart and energy to form new bonds
- What did you learn?
- The more molecules around the higher chance there will be collisions
- Molecules need to be in the right orientation in order to collide
- Thank you for learning about collision theory with me!Power,Barry. Chapter 13-Chemical Kinetics . Chemistry 1051,MUN. Chapter 13 – Chemical Kinetics (Full Slides) - CHEM-1051-001/002/003/004/005 (General Chemistry II) (mun.ca)“Welcome to CK-12 Foundation | CK-12 Foundation.” 4.7 Collision Theory, CK-12, 18 Feb. 2016, www.ck12.org/book/ck-12-chemistry-basic/.Lawson, Peggy, and Stephen Lower. “6.1.6: The Collision theory Peggy Lawson Stephen Lower.” Chemistry LibreTexts, Libretexts, 13Feb. 2023, chem.libretexts.org
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
