Atomic Structure
Storyboard Text
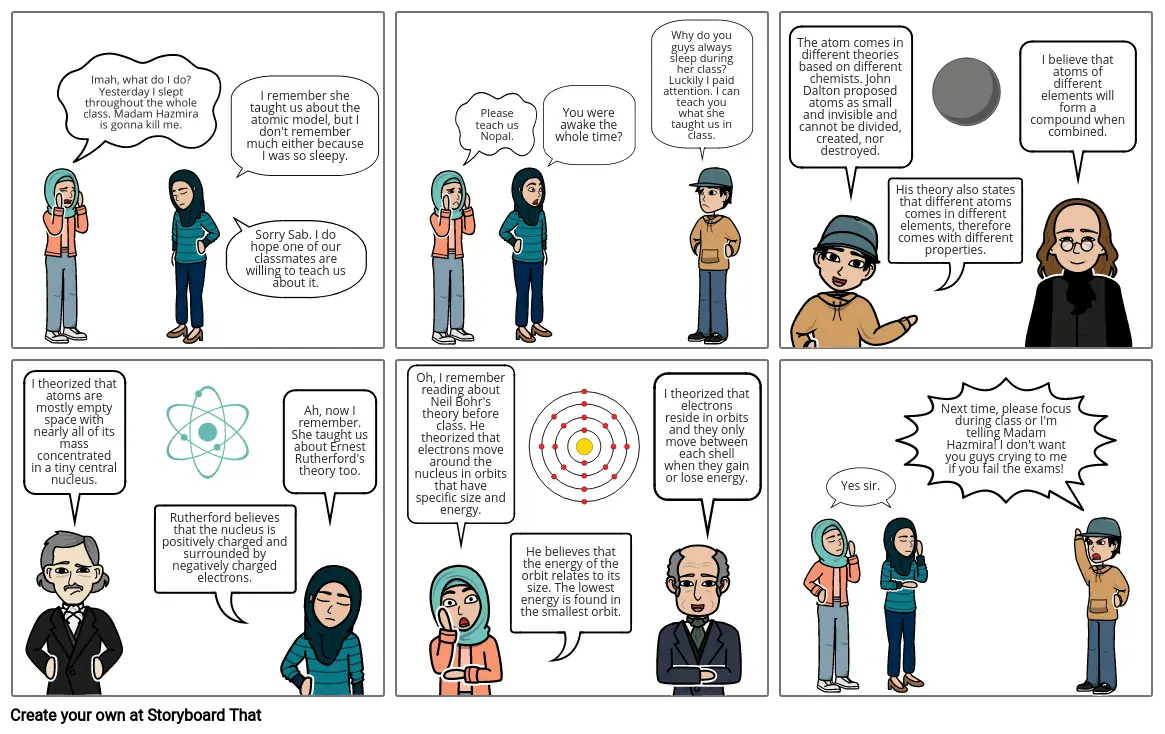
- Imah, what do I do? Yesterday I slept throughout the whole class. Madam Hazmira is gonna kill me.
- Sorry Sab. I do hope one of our classmates are willing to teach us about it.
- I remember she taught us about the atomic model, but I don't remember much either because I was so sleepy.
- Please teach us Nopal.
- You were awake the whole time?
- Why do you guys always sleep during her class? Luckily I paid attention. I can teach you what she taught us in class.
- The atom comes in different theories based on different chemists. John Dalton proposed atoms as small and invisible and cannot be divided, created, nor destroyed.
- His theory also states that different atoms comes in different elements, therefore comes with different properties.
- I believe that atoms of different elements will form a compound when combined.
- I theorized that atoms are mostly empty space with nearly all of its mass concentrated in a tiny central nucleus.
- Rutherford believes that the nucleus is positively charged and surrounded by negatively charged electrons.
- Ah, now I remember. She taught us about Ernest Rutherford's theory too.
- Oh, I remember reading about Neil Bohr's theory before class. He theorized that electrons move around the nucleus in orbits that have specific size and energy.
- He believes that the energy of the orbit relates to its size. The lowest energy is found in the smallest orbit.
- I theorized that electrons reside in orbits and they only move between each shell when they gain or lose energy.
- Yes sir.
- Next time, please focus during class or I'm telling Madam Hazmira! I don't want you guys crying to me if you fail the exams!
Over 30 Million Storyboards Created

