valence electrons
Storyboard Text
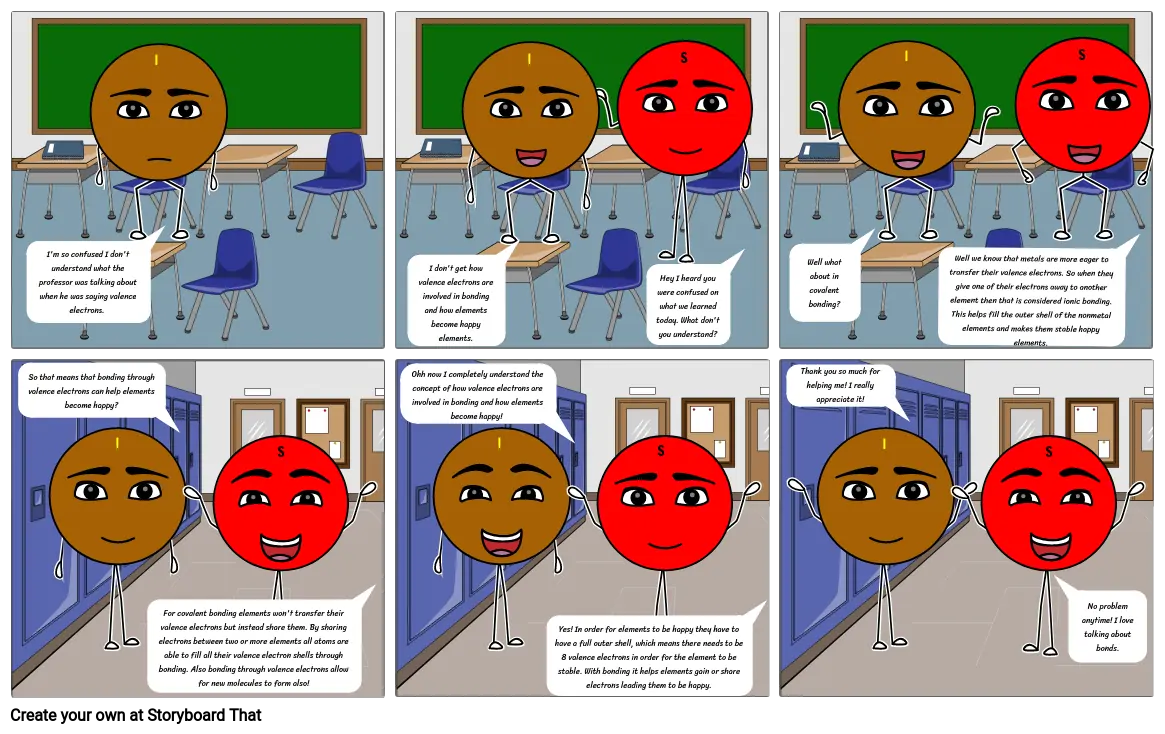
- I'm so confused I don't understand what the professor was talking about when he was saying valence electrons.
- I
- I don't get how valence electrons are involved in bonding and how elements become happy elements.
- I
- Hey I heard you were confused on what we learned today. What don't you understand?
- S
- Well what about in covalent bonding?
- I
- Well we know that metals are more eager to transfer their valence electrons. So when they give one of their electrons away to another element then that is considered ionic bonding. This helps fill the outer shell of the nonmetal elements and makes them stable happy elements.
- S
- So that means that bonding through valence electrons can help elements become happy?
- I
- For covalent bonding elements won't transfer their valence electrons but instead share them. By sharing electrons between two or more elements all atoms are able to fill all their valence electron shells through bonding. Also bonding through valence electrons allow for new molecules to form also!
- S
- Ohh now I completely understand the concept of how valence electrons are involved in bonding and how elements become happy!
- I
- Yes! In order for elements to be happy they have to have a full outer shell, which means there needs to be 8 valence electrons in order for the element to be stable. With bonding it helps elements gain or share electrons leading them to be happy.
- S
- Thank you so much for helping me! I really appreciate it!
- I
- S
- No problem anytime! I love talking about bonds.
Over 30 Million Storyboards Created

