Periodic Trends Cartoon Part 1
Storyboard Text
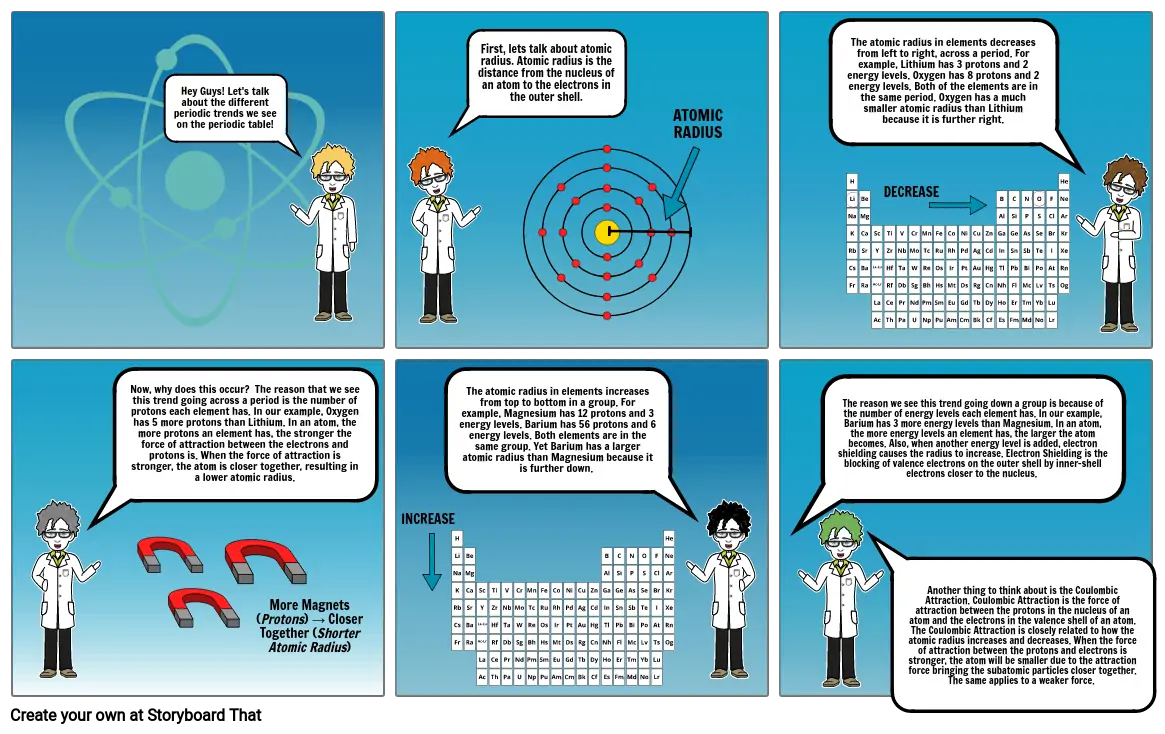
- Hey Guys! Let's talk about the different periodic trends we see on the periodic table!
- First, lets talk about atomic radius. Atomic radius is the distance from the nucleus of an atom to the electrons in the outer shell.
- ATOMIC RADIUS
- The atomic radius in elements decreases from left to right, across a period. For example, Lithium has 3 protons and 2 energy levels. Oxygen has 8 protons and 2 energy levels. Both of the elements are in the same period. Oxygen has a much smaller atomic radius than Lithium because it is further right.
- DECREASE
- Now, why does this occur? The reason that we see this trend going across a period is the number of protons each element has. In our example, Oxygen has 5 more protons than Lithium. In an atom, the more protons an element has, the stronger the force of attraction between the electrons and protons is. When the force of attraction is stronger, the atom is closer together, resulting in a lower atomic radius.
- More Magnets (Protons) → Closer Together (Shorter Atomic Radius)
- INCREASE
- The atomic radius in elements increases from top to bottom in a group. For example, Magnesium has 12 protons and 3 energy levels. Barium has 56 protons and 6 energy levels. Both elements are in the same group. Yet Barium has a larger atomic radius than Magnesium because it is further down.
- The reason we see this trend going down a group is because of the number of energy levels each element has. In our example, Barium has 3 more energy levels than Magnesium. In an atom, the more energy levels an element has, the larger the atom becomes. Also, when another energy level is added, electron shielding causes the radius to increase. Electron Shielding is the blocking of valence electrons on the outer shell by inner-shell electrons closer to the nucleus.
- Another thing to think about is the Coulombic Attraction. Coulombic Attraction is the force of attraction between the protons in the nucleus of an atom and the electrons in the valence shell of an atom. The Coulombic Attraction is closely related to how the atomic radius increases and decreases. When the force of attraction between the protons and electrons is stronger, the atom will be smaller due to the attraction force bringing the subatomic particles closer together. The same applies to a weaker force.
Over 30 Million Storyboards Created

