Atoms
Storyboard Text
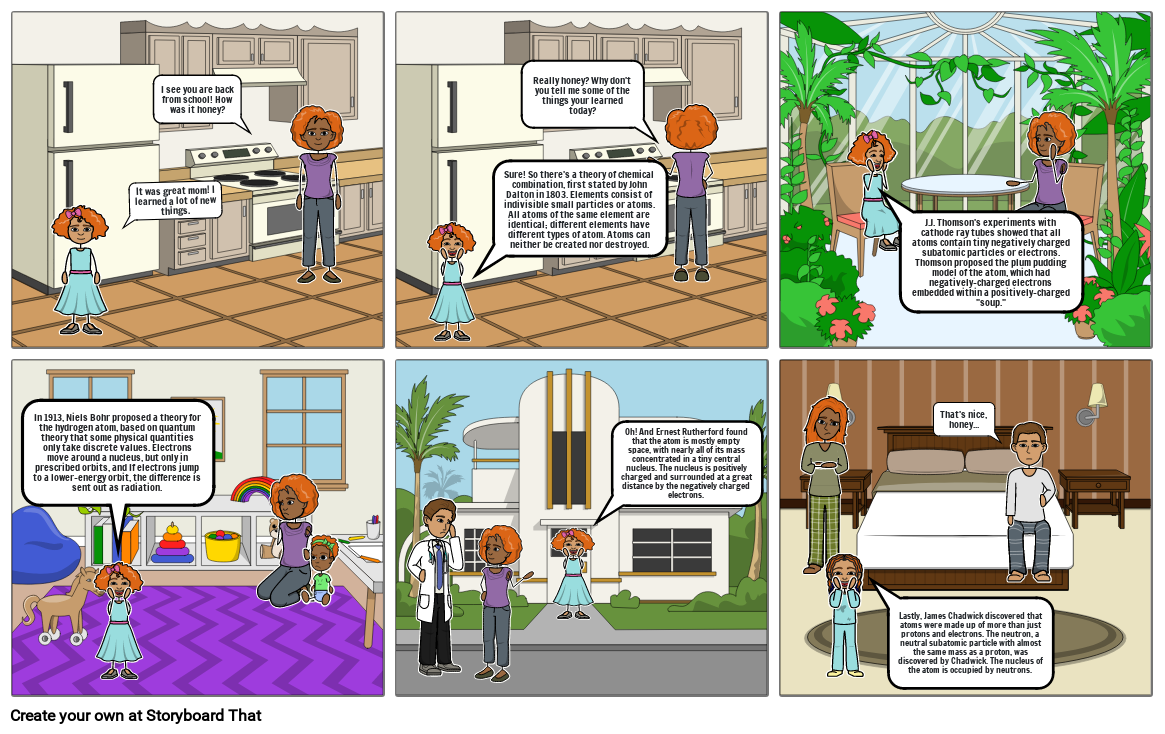
- It was great mom! I learned a lot of new things.
- I see you are back from school! How was it honey?
- Sure! So there's a theory of chemical combination, first stated by John Dalton in 1803. Elements consist of indivisible small particles or atoms. All atoms of the same element are identical; different elements have different types of atom. Atoms can neither be created nor destroyed.
- Really honey? Why don't you tell me some of the things your learned today?
- J.J. Thomson's experiments with cathode ray tubes showed that#160;all atoms contain tiny negatively charged subatomic particles or electrons. Thomson proposed the plum pudding model of the atom, which had negatively-charged electrons embedded within a positively-charged soup.
- In 1913, Niels Bohr proposed a theory for the hydrogen atom, based on quantum theory that some physical quantities only take discrete values.#160;Electrons move around a nucleus, but only in prescribed orbits, and If electrons jump to a lower-energy orbit, the difference is sent out as radiation.
- Oh! And Ernest Rutherford found that#160;the atom is mostly empty space, with nearly all of its mass concentrated in a tiny central nucleus. The nucleus is positively charged and surrounded at a great distance by the negatively charged electrons.
- Lastly, James Chadwick discovered that atoms were made up of more than just protons and electrons. The neutron, a neutral subatomic particle with almost the same mass as a proton, was discovered by Chadwick. The nucleus of the atom is occupied by neutrons.
- That's nice, honey...
Over 30 Million Storyboards Created

