Covalent bonding Pt 2
Storyboard Text
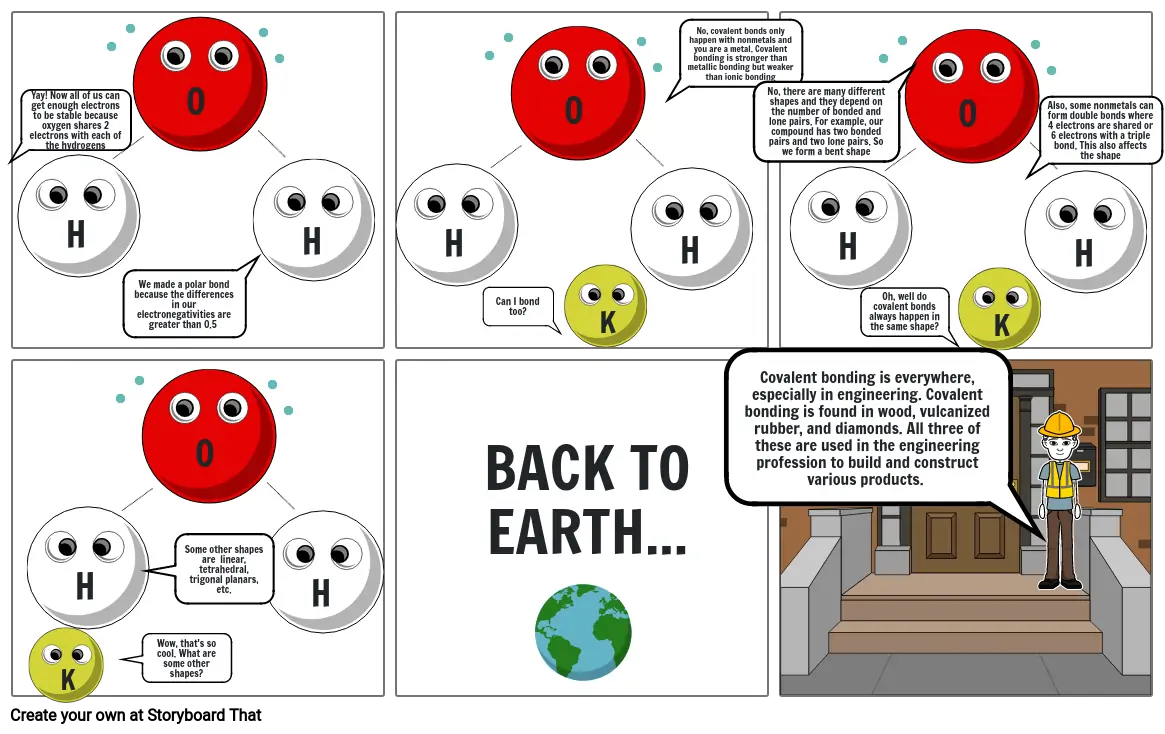
- Yay! Now all of us can get enough electrons to be stable because oxygen shares 2 electrons with each of the hydrogens
- K
- H H H
- H
- H
- Can I bond too?
- O O O
- We made a polar bond because the differences in our electronegativities are greater than 0.5
- O
- K
- H H H
- No, covalent bonds only happen with nonmetals and you are a metal. Covalent bonding is stronger than metallic bonding but weaker than ionic bonding
- Covalent bonding is everywhere, especially in engineering. Covalent bonding is found in wood, vulcanized rubber, and diamonds. All three of these are used in the engineering profession to build and construct various products.
- No, there are many different shapes and they depend on the number of bonded and lone pairs. For example, our compound has two bonded pairs and two lone pairs. So we form a bent shape
- Oh, well do covalent bonds always happen in the same shape?
- K
- Also, some nonmetals can form double bonds where 4 electrons are shared or 6 electrons with a triple bond. This also affects the shape
- Wow, that's so cool. What are some other shapes?
- Some other shapes are linear, tetrahedral, trigonal planars, etc.
- BACK TO EARTH...
Over 30 Million Storyboards Created

