Osmosis Summative EGG
Storyboard Text
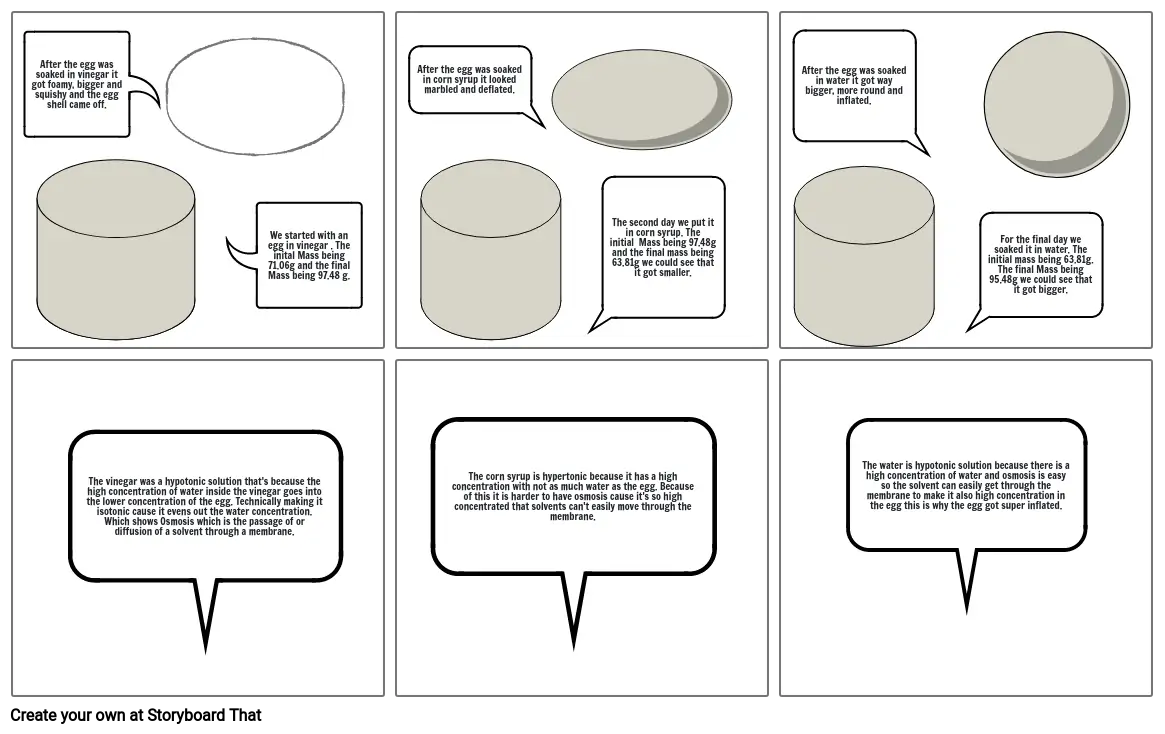
- After the egg was soaked in vinegar it got foamy, bigger and squishy and the egg shell came off.
- We started with an egg in vinegar . The inital Mass being 71.06g and the final Mass being 97.48 g.
- After the egg was soaked in corn syrup it looked marbled and deflated.
- The second day we put it in corn syrup. The initial Mass being 97.48g and the final mass being 63.81g we could see that it got smaller.
- After the egg was soaked in water it got way bigger, more round and inflated.
- For the final day we soaked it in water. The initial mass being 63.81g. The final Mass being 95.48g we could see that it got bigger.
- The vinegar was a hypotonic solution that's because the high concentration of water inside the vinegar goes into the lower concentration of the egg. Technically making it isotonic cause it evens out the water concentration. Which shows Osmosis which is the passage of or diffusion of a solvent through a membrane.
- The corn syrup is hypertonic because it has a high concentration with not as much water as the egg. Because of this it is harder to have osmosis cause it's so high concentrated that solvents can't easily move through the membrane.
- The water is hypotonic solution because there is a high concentration of water and osmosis is easy so the solvent can easily get through the membrane to make it also high concentration in the egg this is why the egg got super inflated.
Over 30 Million Storyboards Created

