History of Atoms
Storyboard Text
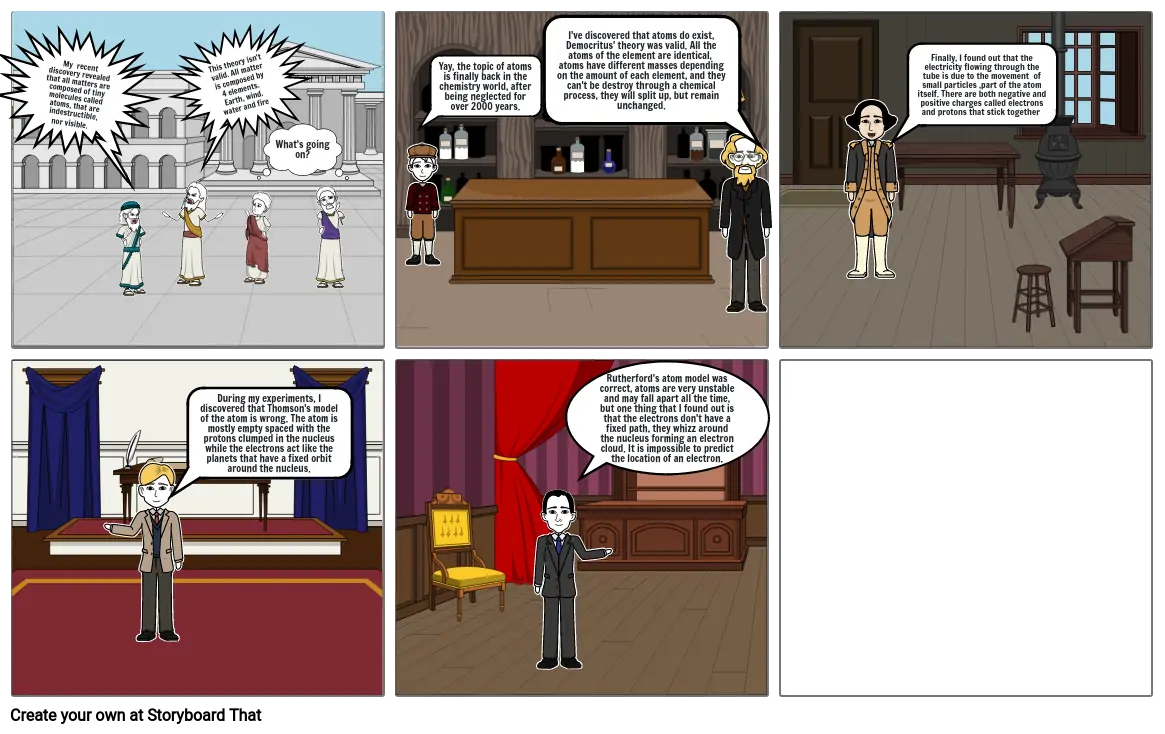
- My recent discovery revealed that all matters are composed of tiny molecules called atoms, that are indestructible, nor visible.
- This theory isn't valid. All matter is composed by 4 elements. Earth, wind. water and fire
- What's going on?
- Yay, the topic of atoms is finally back in the chemistry world, after being neglected for over 2000 years.
- I've discovered that atoms do exist, Democritus' theory was valid. All the atoms of the element are identical, atoms have different masses depending on the amount of each element, and they can't be destroy through a chemical process, they will split up, but remain unchanged.
- Finally, I found out that the electricity flowing through the tube is due to the movement of small particles ,part of the atom itself. There are both negative and positive charges called electrons and protons that stick together
- During my experiments, I discovered that Thomson's model of the atom is wrong. The atom is mostly empty spaced with the protons clumped in the nucleus while the electrons act like the planets that have a fixed orbit around the nucleus.
- Rutherford's atom model was correct, atoms are very unstable and may fall apart all the time, but one thing that I found out is that the electrons don't have a fixed path, they whizz around the nucleus forming an electron cloud. It is impossible to predict the location of an electron.
Over 30 Million Storyboards Created

