Comic Strip
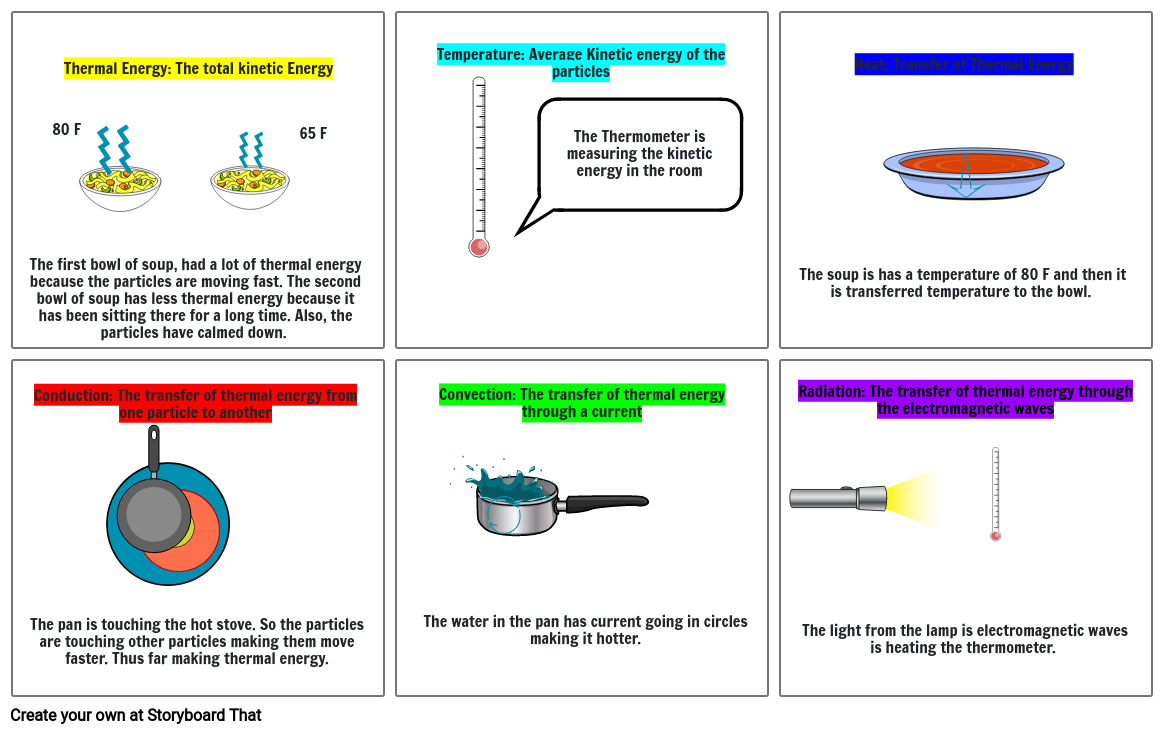
Thermal Energy: The total kinetic Energy
Temperature: Average Kinetic energy of the particles
Heat: Transfer of Thermal Energy
Conduction: The transfer of thermal energy from one particle to another
Convection: The transfer of thermal energy through a current
Radiation: The transfer of thermal energy through the electromagnetic waves
The first bowl of soup, had a lot of thermal energy because the particles are moving fast. The second bowl of soup has less thermal energy because it has been sitting there for a long time. Also, the particles have calmed down.
80 F
65F
The soup is has a temperature of 80 F and then it is transferred temperature to the bowl.
The Thermometeris measuring the kinetic energy in the room
The pan is touching the hot stove. So the particles are touching other particles making them move faster. Thus far making thermal energy.
The water in the pan has current going in circles making it hotter.
The light from the lamp is electromagnetic waves is heating the thermometer.
Thermal Energy: The total kinetic Energy
Temperature: Average Kinetic energy of the particles
Heat: Transfer of Thermal Energy
Conduction: The transfer of thermal energy from one particle to another
Convection: The transfer of thermal energy through a current
Radiation: The transfer of thermal energy through the electromagnetic waves
The first bowl of soup, had a lot of thermal energy because the particles are moving fast. The second bowl of soup has less thermal energy because it has been sitting there for a long time. Also, the particles have calmed down.
80 F
65F
The soup is has a temperature of 80 F and then it is transferred temperature to the bowl.
The Thermometeris measuring the kinetic energy in the room
The pan is touching the hot stove. So the particles are touching other particles making them move faster. Thus far making thermal energy.
The water in the pan has current going in circles making it hotter.
The light from the lamp is electromagnetic waves is heating the thermometer.
Thermal Energy: The total kinetic Energy
Temperature: Average Kinetic energy of the particles
Heat: Transfer of Thermal Energy
Conduction: The transfer of thermal energy from one particle to another
Convection: The transfer of thermal energy through a current
Radiation: The transfer of thermal energy through the electromagnetic waves
The first bowl of soup, had a lot of thermal energy because the particles are moving fast. The second bowl of soup has less thermal energy because it has been sitting there for a long time. Also, the particles have calmed down.
80 F
65F
The soup is has a temperature of 80 F and then it is transferred temperature to the bowl.
The Thermometeris measuring the kinetic energy in the room
The pan is touching the hot stove. So the particles are touching other particles making them move faster. Thus far making thermal energy.
The water in the pan has current going in circles making it hotter.
The light from the lamp is electromagnetic waves is heating the thermometer.
Thermal Energy: The total kinetic Energy
Temperature: Average Kinetic energy of the particles
Heat: Transfer of Thermal Energy
Conduction: The transfer of thermal energy from one particle to another
Convection: The transfer of thermal energy through a current
Radiation: The transfer of thermal energy through the electromagnetic waves
The first bowl of soup, had a lot of thermal energy because the particles are moving fast. The second bowl of soup has less thermal energy because it has been sitting there for a long time. Also, the particles have calmed down.
80 F
65F
The soup is has a temperature of 80 F and then it is transferred temperature to the bowl.
The Thermometeris measuring the kinetic energy in the room
The pan is touching the hot stove. So the particles are touching other particles making them move faster. Thus far making thermal energy.
The water in the pan has current going in circles making it hotter.
The light from the lamp is electromagnetic waves is heating the thermometer.
Thermal Energy: The total kinetic Energy
Temperature: Average Kinetic energy of the particles
Heat: Transfer of Thermal Energy
Conduction: The transfer of thermal energy from one particle to another
Convection: The transfer of thermal energy through a current
Radiation: The transfer of thermal energy through the electromagnetic waves
The first bowl of soup, had a lot of thermal energy because the particles are moving fast. The second bowl of soup has less thermal energy because it has been sitting there for a long time. Also, the particles have calmed down.
80 F
65F
The soup is has a temperature of 80 F and then it is transferred temperature to the bowl.
The Thermometeris measuring the kinetic energy in the room
The pan is touching the hot stove. So the particles are touching other particles making them move faster. Thus far making thermal energy.
The water in the pan has current going in circles making it hotter.
The light from the lamp is electromagnetic waves is heating the thermometer.
Thermal Energy: The total kinetic Energy
Temperature: Average Kinetic energy of the particles
Heat: Transfer of Thermal Energy
Conduction: The transfer of thermal energy from one particle to another
Convection: The transfer of thermal energy through a current
Radiation: The transfer of thermal energy through the electromagnetic waves
The first bowl of soup, had a lot of thermal energy because the particles are moving fast. The second bowl of soup has less thermal energy because it has been sitting there for a long time. Also, the particles have calmed down.
80 F
65F
The soup is has a temperature of 80 F and then it is transferred temperature to the bowl.
The Thermometeris measuring the kinetic energy in the room
The pan is touching the hot stove. So the particles are touching other particles making them move faster. Thus far making thermal energy.
The water in the pan has current going in circles making it hotter.
The light from the lamp is electromagnetic waves is heating the thermometer.
Thermal Energy: The total kinetic Energy
Temperature: Average Kinetic energy of the particles
Heat: Transfer of Thermal Energy
Conduction: The transfer of thermal energy from one particle to another
Convection: The transfer of thermal energy through a current
Radiation: The transfer of thermal energy through the electromagnetic waves
The first bowl of soup, had a lot of thermal energy because the particles are moving fast. The second bowl of soup has less thermal energy because it has been sitting there for a long time. Also, the particles have calmed down.
80 F
65F
The soup is has a temperature of 80 F and then it is transferred temperature to the bowl.
The Thermometeris measuring the kinetic energy in the room
The pan is touching the hot stove. So the particles are touching other particles making them move faster. Thus far making thermal energy.
The water in the pan has current going in circles making it hotter.
The light from the lamp is electromagnetic waves is heating the thermometer.
Storyboard Text
- Thermal Energy: The total kinetic Energy
- The first bowl of soup, had a lot of thermal energy because the particles are moving fast. The second bowl of soup has less thermal energy because it has been sitting there for a long time. Also, the particles have calmed down.
- 80 F
- 65 F
- Temperature: Average Kinetic energy of the particles
- The Thermometer is measuring the kinetic energy in the room
- Heat: Transfer of Thermal Energy
- The soup is has a temperature of 80 F and then it is transferred temperature to the bowl.
- The pan is touching the hot stove. So the particles are touching other particles making them move faster. Thus far making thermal energy.
- Conduction: The transfer of thermal energy from one particle to another
- Convection: The transfer of thermal energy through a current
- The water in the pan has current going in circles making it hotter.
- The light from the lamp is electromagnetic waves is heating the thermometer.
- Radiation: The transfer of thermal energy through the electromagnetic waves



