Le Chatelier Principal
Storyboard Text
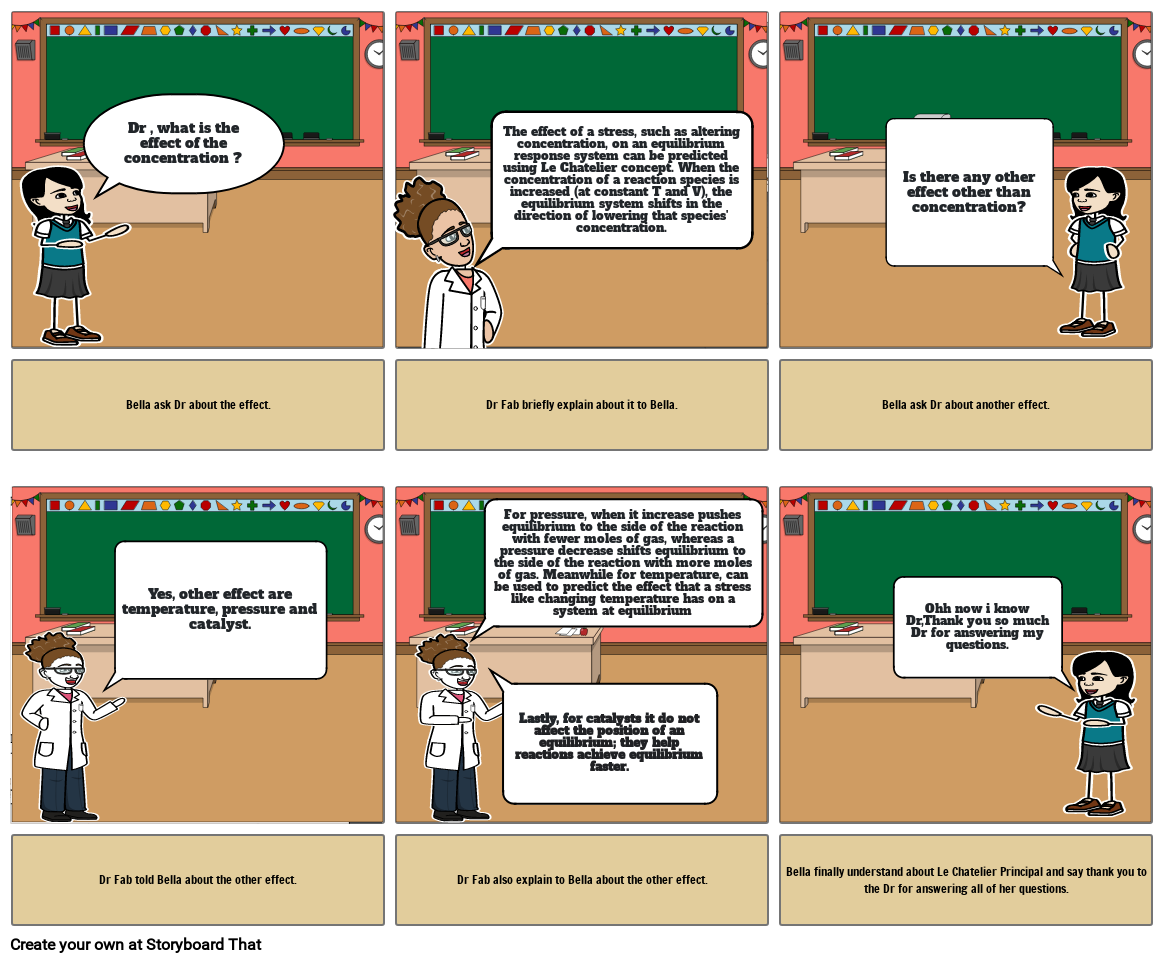
- Dr , what is the effect of the concentration ?
- The effect of a stress, such as altering concentration, on an equilibrium response system can be predicted using Le Chatelier concept. When the concentration of a reaction species is increased (at constant T and V), the equilibrium system shifts in the direction of lowering that species' concentration.
- Is there any other effect other than concentration?
- Bella ask Dr about the effect.
- Yes, other effect are temperature, pressure and catalyst.
- Dr Fab briefly explain about it to Bella.
- For pressure, when it increase pushes equilibrium to the side of the reaction with fewer moles of gas, whereas a pressure decrease shifts equilibrium to the side of the reaction with more moles of gas. Meanwhile for temperature, can be used to predict the effect that a stress like changing temperature has on a system at equilibrium
- Lastly, for catalysts it do not affect the position of an equilibrium; they help reactions achieve equilibrium faster.
- Bella ask Dr about another effect.
- Ohh now i know Dr,Thank you so much Dr for answering my questions.
- Dr Fab told Bella about the other effect.
- Dr Fab also explain to Bella about the other effect.
- Bella finally understand about Le Chatelier Principal and say thank you to the Dr for answering all of her questions.
Over 30 Million Storyboards Created

