Chemistry Comic strip
Storyboard Text
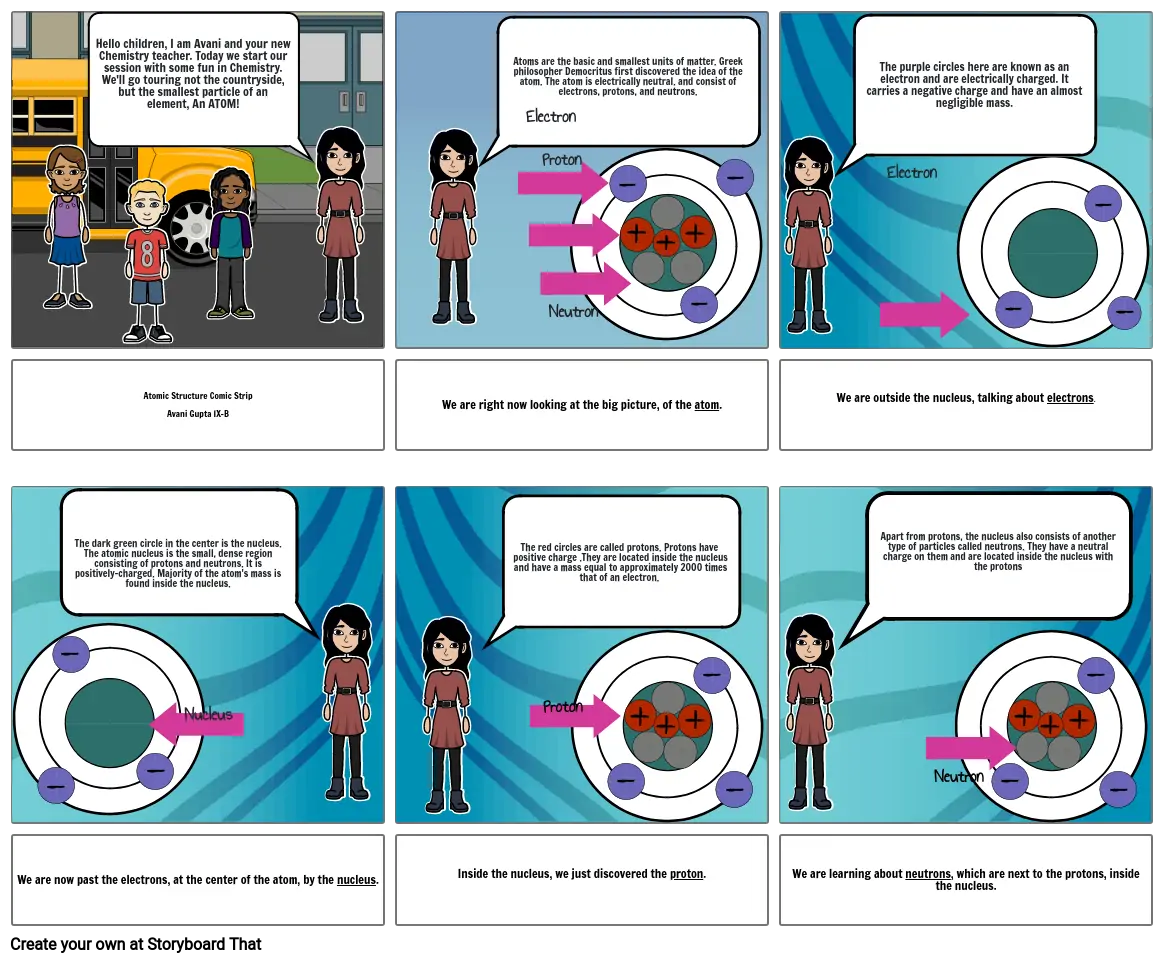
- Hello children, I am Avani and your new Chemistry teacher. Today we start our session with some fun in Chemistry. We'll go touring not the countryside, but the smallest particle of an element, An ATOM!
- Atoms are the basic and smallest units of matter. Greek philosopher Democritus first discovered the idea of the atom. The atom is electrically neutral. and consist of electrons, protons, and neutrons.
- Electron
- Proton
- Neutron
- The purple circles here are known as an electron and are electrically charged. It carries a negative charge and have an almost negligible mass.
- Electron
- Atomic Structure Comic StripAvani Gupta IX-B
- The dark green circle in the center is the nucleus. The atomic nucleus is the small, dense region consisting of protons and neutrons. It is positively-charged. Majority of the atom's mass is found inside the nucleus.
- We are right now looking at the big picture, of the atom.
- The red circles are called protons. Protons have positive charge .They are located inside the nucleus and have a mass equal to approximately 2000 times that of an electron.
- Proton
- We are outside the nucleus, talking about electrons.
- Apart from protons, the nucleus also consists of another type of particles called neutrons. They have a neutral charge on them and are located inside the nucleus with the protons
- We are now past the electrons, at the center of the atom, by the nucleus.
- Nucleus
- Inside the nucleus, we just discovered the proton.
- We are learning about neutrons, which are next to the protons, inside the nucleus.
- Neutron
Over 30 Million Storyboards Created

