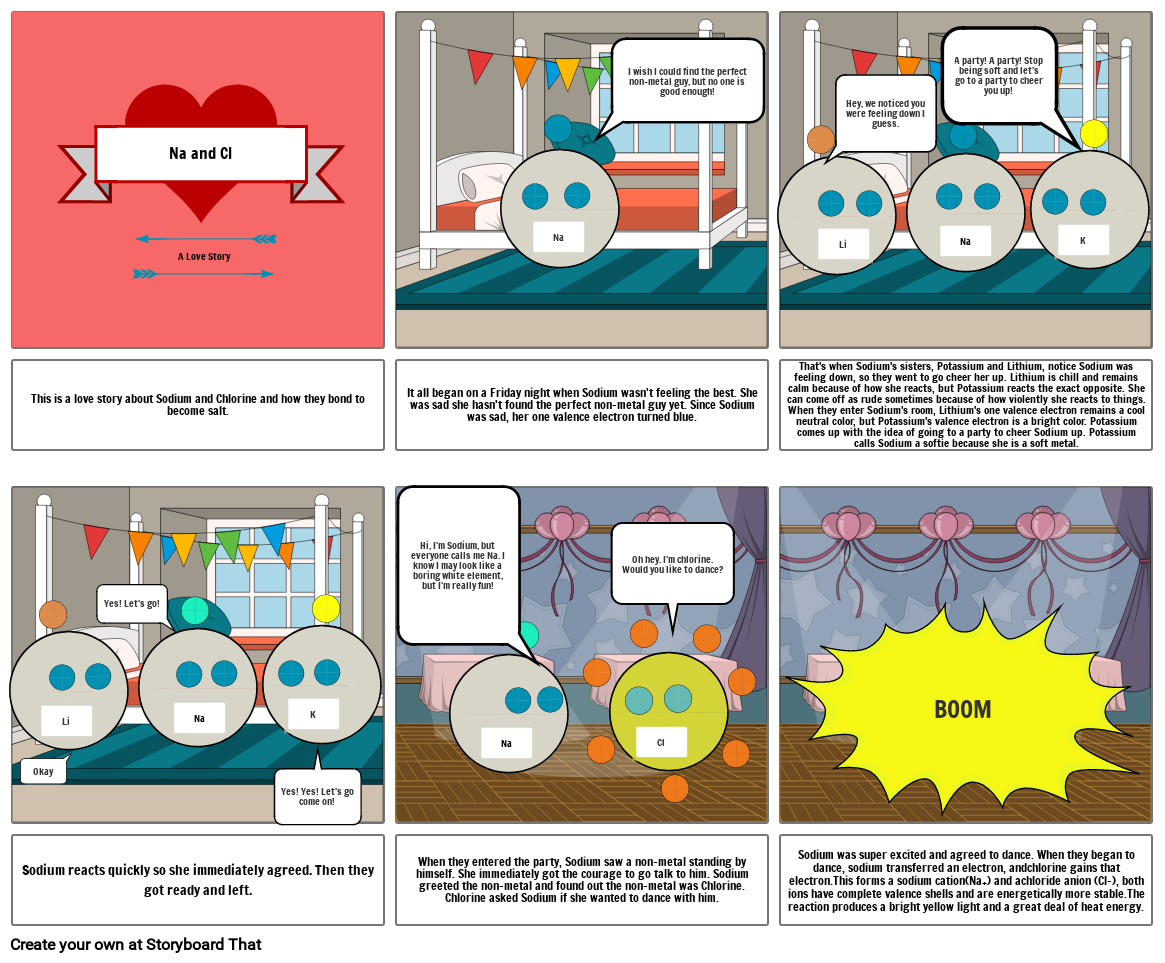
Sodium chloride
Storyboard Text
- Na and Cl
- A Love Story
- Na
- I wish I could find the perfect non-metal guy, but no one is good enough!
- Li
- Hey, we noticed you were feeling down I guess.
- Na
- A party! A party! Stop being soft and let's go to a party to cheer you up!
- K
- This is a love story about Sodium and Chlorine and how they bond to become salt.
- Yes! Let's go!
- K
- It all began on a Friday night when Sodium wasn't feeling the best. She was sad she hasn't found the perfect non-metal guy yet. Since Sodium was sad, her one valence electron turned blue.
- Hi, I'm Sodium, but everyone calls me Na. I know I may look like a boring white element, but I'm really fun!
- Oh hey. I'm chlorine. Would you like to dance?
- That's when Sodium's sisters, Potassium and Lithium, notice Sodium was feeling down, so they went to go cheer her up. Lithium is chill and remains calm because of how she reacts, but Potassium reacts the exact opposite. She can come off as rude sometimes because of how violently she reacts to things. When they enter Sodium's room, Lithium's one valence electron remains a cool neutral color, but Potassium's valence electron is a bright color. Potassium comes up with the idea of going to a party to cheer Sodium up. Potassium calls Sodium a softie because she is a soft metal.
- BOOM
- Sodium reacts quickly so she immediately agreed. Then they got ready and left.
- Okay
- Li
- Na
- Yes! Yes! Let's go come on!
- When they entered the party, Sodium saw a non-metal standing by himself. She immediately got the courage to go talk to him. Sodium greeted the non-metal and found out the non-metal was Chlorine. Chlorine asked Sodium if she wanted to dance with him.
- Na
- Cl
- Sodium was super excited and agreed to dance. When they began to dance, sodium transferred an electron, andchlorine gains that electron.This forms a sodium cation(Na+) and achloride anion (Cl-), both ions have complete valence shells and are energetically more stable.The reaction produces a bright yellow light and a great deal of heat energy.
Over 30 Million Storyboards Created

