Thermal energy Comic strip
Storyboard Text
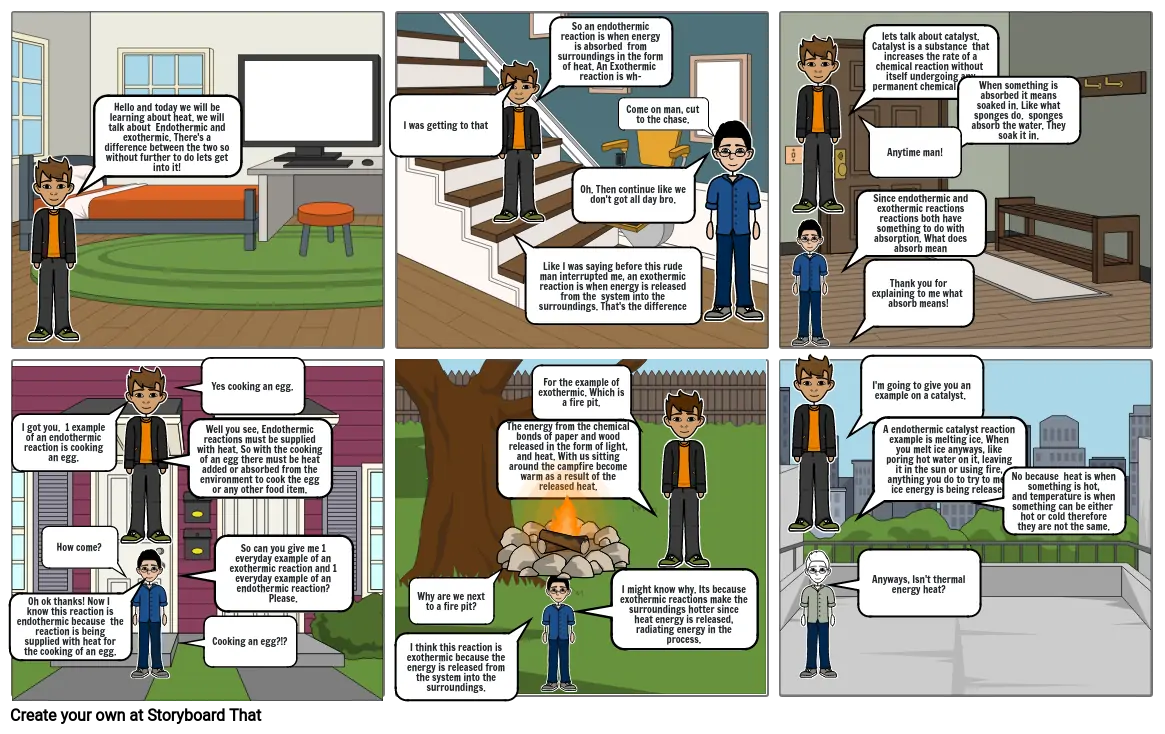
- Hello and today we will be learning about heat. we will talk about Endothermic and exothermic. There's a difference between the two so without further to do lets get into it!
- I was getting to that
- Like I was saying before this rude man interrupted me, an exothermic reaction is when energy is released from the system into the surroundings. That's the difference
- So an endothermic reaction is when energy is absorbed from surroundings in the form of heat. An Exothermic reaction is wh-
- Oh. Then continue like we don't got all day bro.
- Come on man, cut to the chase.
- Since endothermic and exothermic reactions reactions both have something to do with absorption. What does absorb mean
- lets talk about catalyst. Catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change
- Thank you for explaining to me what absorb means!
- Anytime man!
- When something is absorbed it means soaked in. Like what sponges do. sponges absorb the water. They soak it in.
- Oh ok thanks! Now I know this reaction is endothermic because the reaction is being supplied with heat for the cooking of an egg.
- I got you. 1 example of an endothermic reaction is cooking an egg.
- How come?
- Well you see, Endothermic reactions must be supplied with heat. So with the cooking of an egg there must be heat added or absorbed from the environment to cook the egg or any other food item.
- Yes cooking an egg.
- So can you give me 1 everyday example of an exothermic reaction and 1 everyday example of an endothermic reaction? Please.
- Cooking an egg?!?
- I think this reaction is exothermic because the energy is released from the system into the surroundings.
- Why are we next to a fire pit?
- The energy from the chemical bonds of paper and wood released in the form of light, and heat. With us sitting around the campfire become warm as a result of the released heat.
- For the example of exothermic. Which is a fire pit.
- I might know why. Its because exothermic reactions make the surroundings hotter since heat energy is released, radiating energy in the process.
- Anyways, Isn't thermal energy heat?
- I'm going to give you an example on a catalyst.
- A endothermic catalyst reaction example is melting ice. When you melt ice anyways, like poring hot water on it, leaving it in the sun or using fire, anything you do to try to melt ice energy is being released
- No because heat is when something is hot, and temperature is when something can be either hot or cold therefore they are not the same.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!

