History of the Atom
Storyboard Description
11/6/21 Work
Storyboard Text
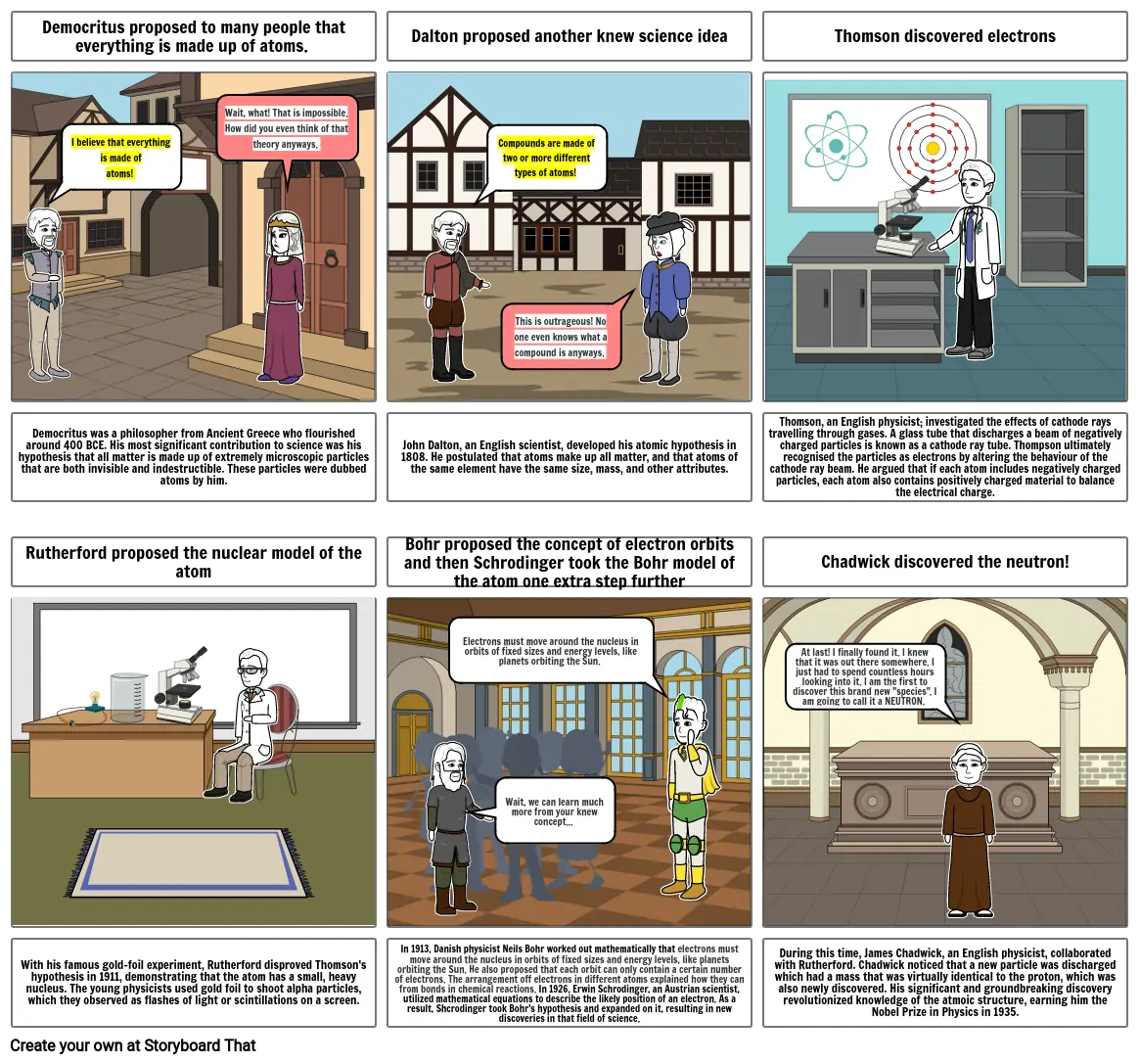
- Democritus proposed to many people that everything is made up of atoms.
- I believe that everything is made of atoms!
- Wait, what! That is impossible. How did you even think of that theory anyways.
- Dalton proposed another knew science idea
- Compounds are made of two or more different types of atoms!
- This is outrageous! No one even knows what a compound is anyways.
- Thomson discovered electrons
- Democritus was a philosopher from Ancient Greece who flourished around 400 BCE. His most significant contribution to science was his hypothesis that all matter is made up of extremely microscopic particles that are both invisible and indestructible. These particles were dubbed atoms by him.
- Rutherford proposed the nuclear model of the atom
- John Dalton, an English scientist, developed his atomic hypothesis in 1808. He postulated that atoms make up all matter, and that atoms of the same element have the same size, mass, and other attributes.
- Bohr proposed the concept of electron orbits and then Schrodinger took the Bohr model of the atom one extra step further
- Electrons must move around the nucleus in orbits of fixed sizes and energy levels, like planets orbiting the Sun.
- Thomson, an English physicist; investigated the effects of cathode rays travelling through gases. A glass tube that discharges a beam of negatively charged particles is known as a cathode ray tube. Thompson ultimately recognised the particles as electrons by altering the behaviour of the cathode ray beam. He argued that if each atom includes negatively charged particles, each atom also contains positively charged material to balance the electrical charge.
- Chadwick discovered the neutron!
- At last! I finally found it. I knew that it was out there somewhere. I just had to spend countless hours looking into it. I am the first to discover this brand new "species". I am going to call it a NEUTRON.
- With his famous gold-foil experiment, Rutherford disproved Thomson's hypothesis in 1911, demonstrating that the atom has a small, heavy nucleus. The young physicists used gold foil to shoot alpha particles, which they observed as flashes of light or scintillations on a screen.
- In 1913, Danish physicist Neils Bohr worked out mathematically that electrons must move around the nucleus in orbits of fixed sizes and energy levels, like planets orbiting the Sun. He also proposed that each orbit can only contain a certain number of electrons. The arrangement off electrons in different atoms explained how they can from bonds in chemical reactions. In 1926, Erwin Schrodinger, an Austrian scientist, utilized mathematical equations to describe the likely position of an electron. As a result, Shcrodinger took Bohr's hypothesis and expanded on it, resulting in new discoveries in that field of science.
- Wait, we can learn much more from your knew concept...
- During this time, James Chadwick, an English physicist, collaborated with Rutherford. Chadwick noticed that a new particle was discharged which had a mass that was virtually identical to the proton, which was also newly discovered. His significant and groundbreaking discovery revolutionized knowledge of the atmoic structure, earning him the Nobel Prize in Physics in 1935.
Over 30 Million Storyboards Created

