Combustion
Storyboard Text
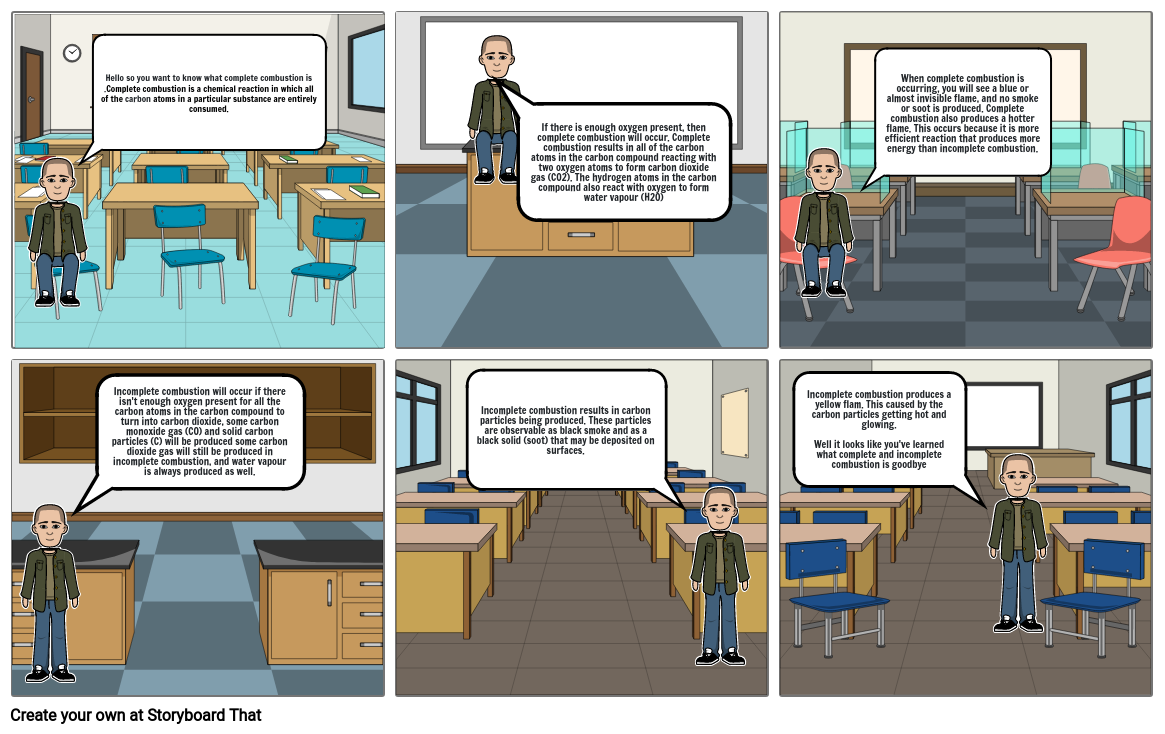
- Hello so you want to know what complete combustion is .Complete combustion is a chemical reaction in which all of the carbon atoms in a particular substance are entirely consumed.
- If there is enough oxygen present, then complete combustion will occur. Complete combustion results in all of the carbon atoms in the carbon compound reacting with two oxygen atoms to form carbon dioxide gas (CO2). The hydrogen atoms in the carbon compound also react with oxygen to form water vapour (H2O)
- When complete combustion is occurring, you will see a blue or almost invisible flame, and no smoke or soot is produced. Complete combustion also produces a hotter flame. This occurs because it is more efficient reaction that produces more energy than incomplete combustion.
- Incomplete combustion will occur if there isn't enough oxygen present for all the carbon atoms in the carbon compound to turn into carbon dioxide, some carbon monoxide gas (CO) and solid carbon particles (C) will be produced some carbon dioxide gas will still be produced in incomplete combustion, and water vapour is always produced as well.
- Incomplete combustion results in carbon particles being produced. These particles are observable as black smoke and as a black solid (soot) that may be deposited on surfaces.
- Incomplete combustion produces a yellow flam. This caused by the carbon particles getting hot and glowing. Well it looks like you've learned what complete and incomplete combustion is goodbye
Over 30 Million Storyboards Created

