hot cold air balloon charles law
Storyboard Text
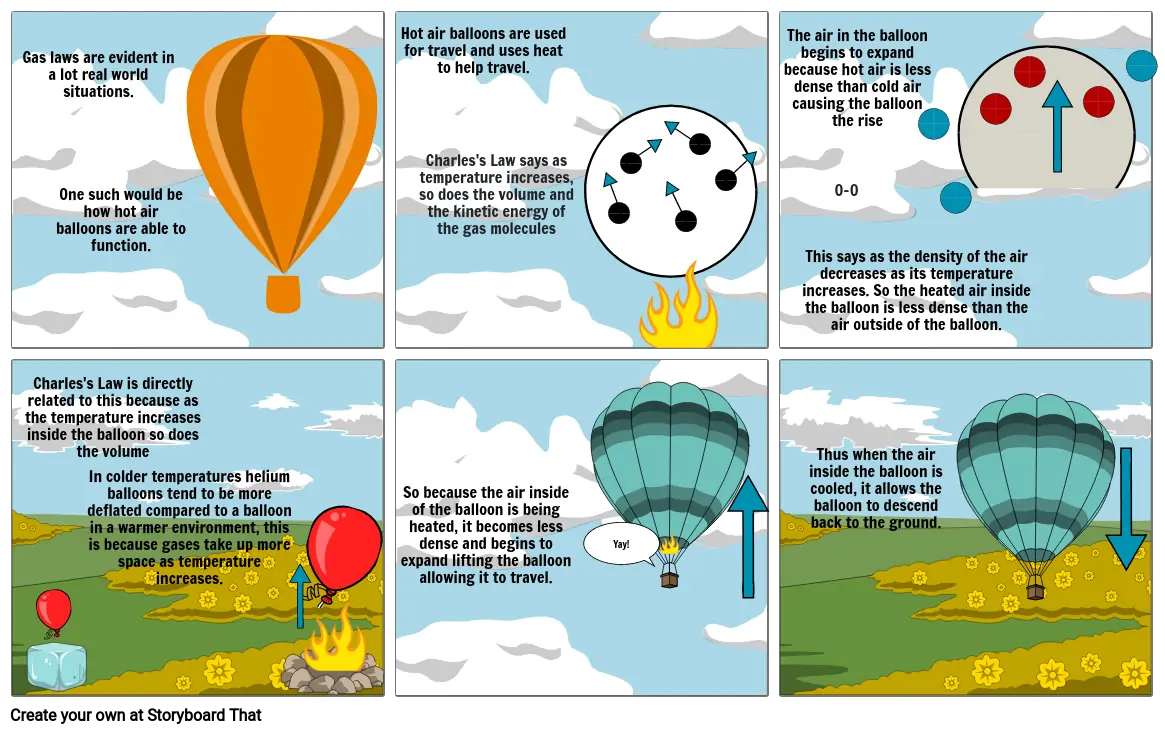
- Gas laws are evident in a lot real world situations.
- One such would be how hot air balloons are able to function.
- Hot air balloons are used for travel and uses heat to help travel.
- Charles's Law says as temperature increases, so does the volume and the kinetic energy of the gas molecules
- 0-0
- The air in the balloon begins to expand because hot air is less dense than cold air causing the balloon the rise
- This says as the density of the air decreases as its temperature increases. So the heated air inside the balloon is less dense than the air outside of the balloon.
- Charles's Law is directly related to this because as the temperature increases inside the balloon so does the volume
- In colder temperatures helium balloons tend to be more deflated compared to a balloon in a warmer environment, this is because gases take up more space as temperature increases.
- So because the air inside of the balloon is being heated, it becomes less dense and begins to expand lifting the balloon allowing it to travel.
- Yay!
- Thus when the air inside the balloon is cooled, it allows the balloon to descend back to the ground.
Over 30 Million Storyboards Created

