Unknown Story
Snemalna Knjiga Besedilo
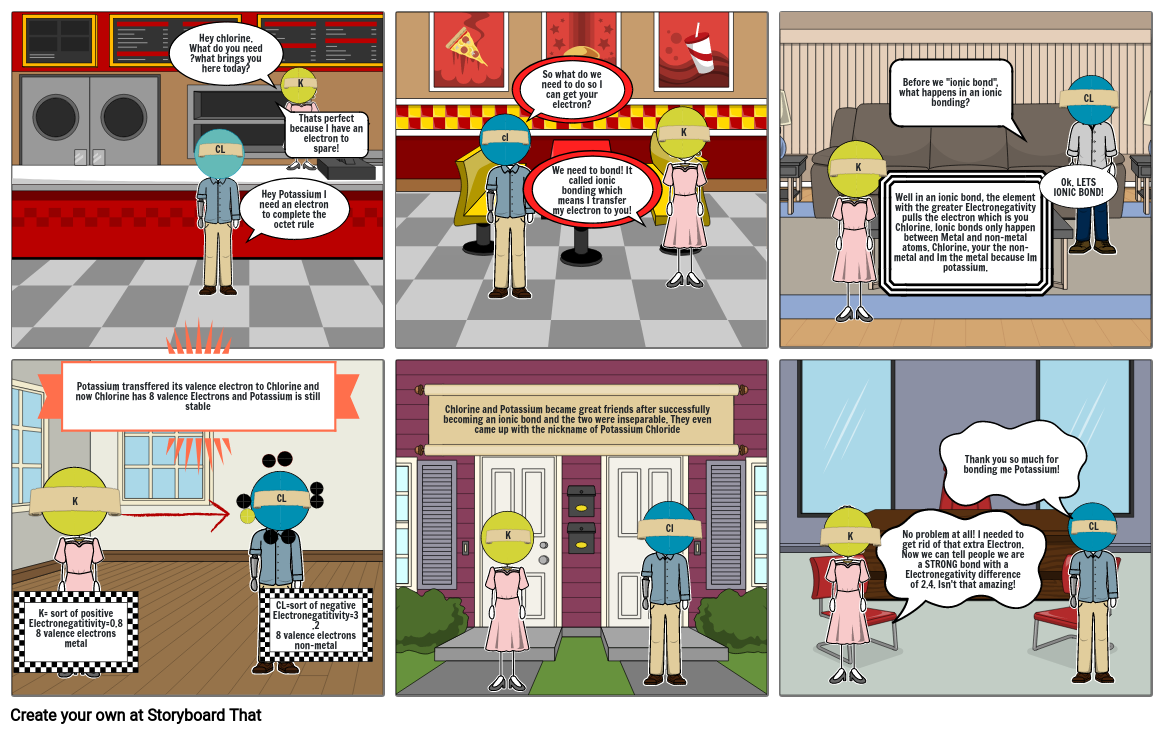
- Potassium transffered its valence electron to Chlorine and now Chlorine has 8 valence Electrons and Potassium is still stable
- Hey chlorine. What do you need ?what brings you here today?
- CL
- Hey Potassium I need an electron to complete the octet rule
- Thats perfect because I have an electron to spare!
- K
- cl
- So what do we need to do so I can get your electron?
- We need to bond! It called ionic bonding which means I transfer my electron to you!
- K
- K
- Well in an ionic bond, the element with the greater Electronegativity pulls the electron which is you Chlorine. Ionic bonds only happen between Metal and non-metal atoms. Chlorine, your the non-metal and Im the metal because Im potassium.
- Before we "ionic bond", what happens in an ionic bonding?
- Ok. LETS IONIC BOND!
- CL
- K= sort of positiveElectronegatitivity=0.88 valence electronsmetal
- K
- CL
- CL=sort of negativeElectronegatitivity=3.28 valence electronsnon-metal
- Chlorine and Potassium became great friends after successfully becoming an ionic bond and the two were inseparable. They even came up with the nickname of Potassium Chloride
- K
- Cl
- K
- No problem at all! I needed to get rid of that extra Electron. Now we can tell people we are a STRONG bond with a Electronegativity difference of 2.4. Isn't that amazing!
- Thank you so much for bonding me Potassium!
- CL
Ustvarjenih več kot 30 milijonov snemalnih knjig

