Atom: Bohr's Model
Snemalna Knjiga Besedilo
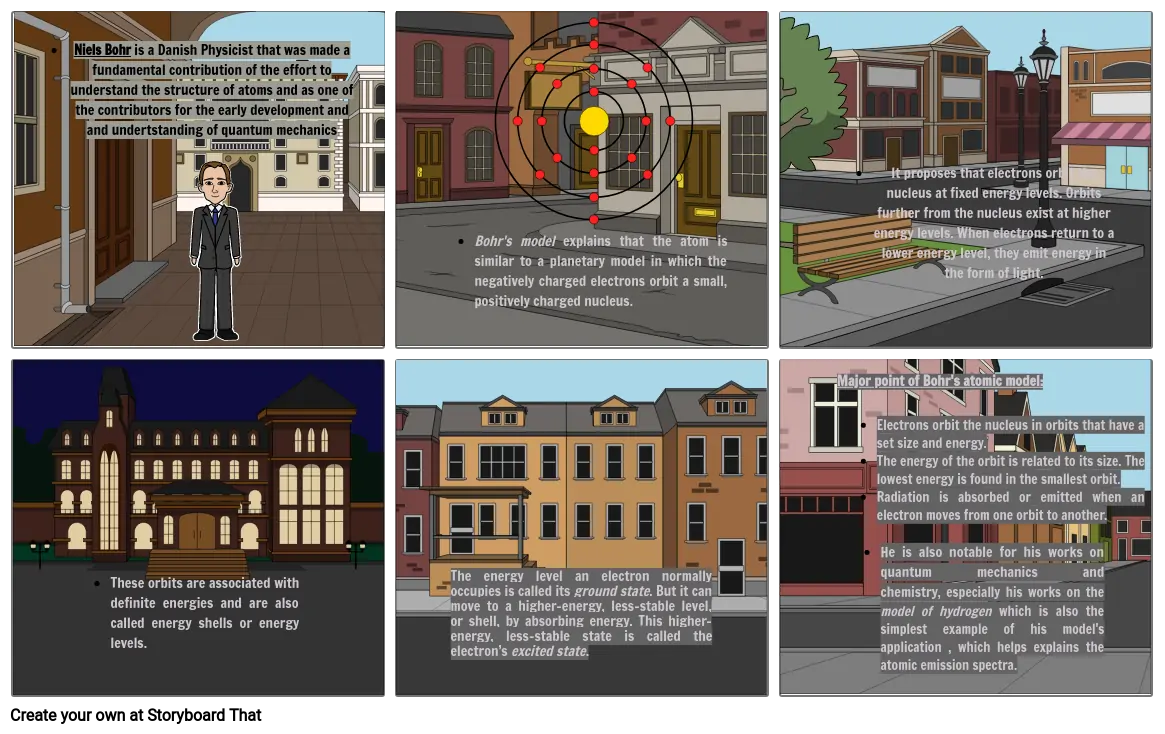
- Niels Bohr is a Danish Physicist that was made a fundamental contribution of the effort to understand the structure of atoms and as one of the contributors for the early development and and undertstanding of quantum mechanics
- Bohr's model explains that the atom is similar to a planetary model in which the negatively charged electrons orbit a small, positively charged nucleus.
- It proposes that electrons orbit the nucleus at fixed energy levels. Orbits further from the nucleus exist at higher energy levels. When electrons return to a lower energy level, they emit energy in the form of light.
- These orbits are associated with definite energies and are also called energy shells or energy levels.
- The energy level an electron normally occupies is called its ground state. But it can move to a higher-energy, less-stable level, or shell, by absorbing energy. This higher-energy, less-stable state is called the electron’s excited state.
- Major point of Bohr's atomic model:Electrons orbit the nucleus in orbits that have a set size and energy.The energy of the orbit is related to its size. The lowest energy is found in the smallest orbit.Radiation is absorbed or emitted when an electron moves from one orbit to another.
- He is also notable for his works on quantum mechanics and chemistry, especially his works on the model of hydrogen which is also the simplest example of his model's application , which helps explains the atomic emission spectra.
Ustvarjenih več kot 30 milijonov snemalnih knjig

