BONDING AND PHYSICAL PROPERTIES OF MATTER
Snemalna Knjiga Besedilo
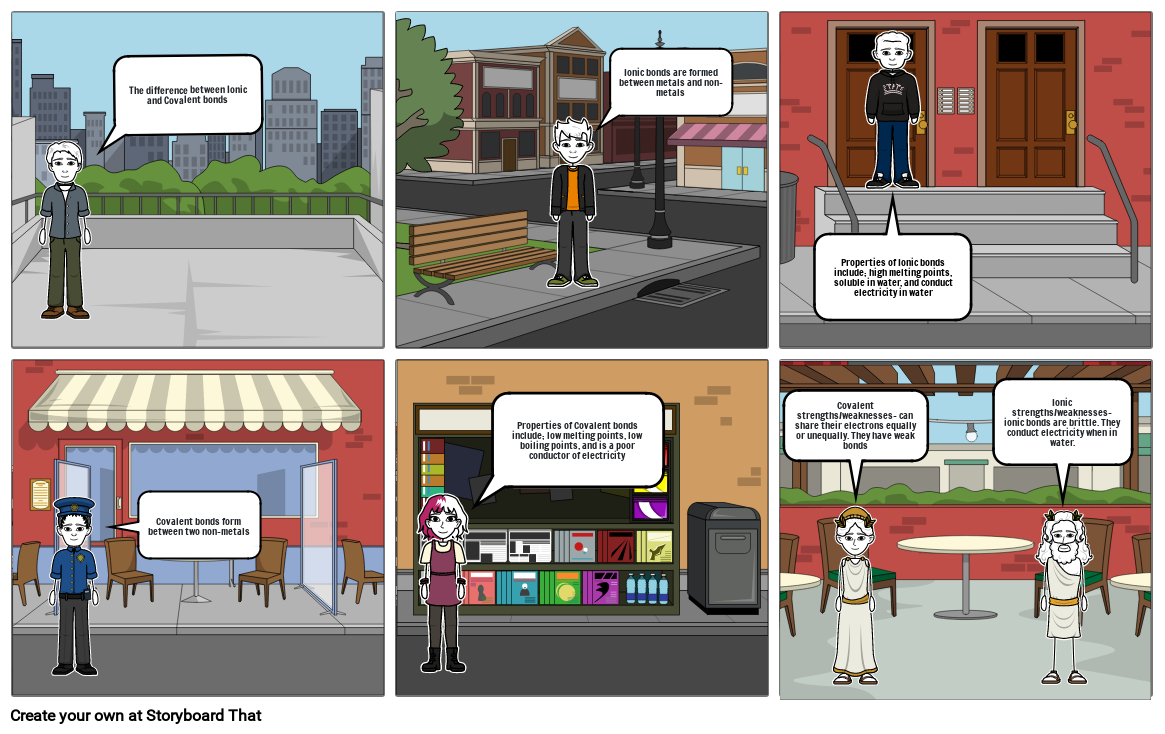
- The difference between Ionic and Covalent bonds
- Ionic bonds are formed between metals and non-metals
- Properties of Ionic bonds include; high melting points, soluble in water, and conduct electricity in water
- Covalent bonds form between two non-metals
- Properties of Covalent bonds include; low melting points, low boiling points, and is a poor conductor of electricity
- Covalent strengths/weaknesses- can share their electrons equally or unequally. They have weak bonds
- Ionic strengths/weaknesses- ionic bonds are brittle. They conduct electricity when in water.
Ustvarjenih več kot 30 milijonov snemalnih knjig

