Boyle's Law
Snemalna Knjiga Besedilo
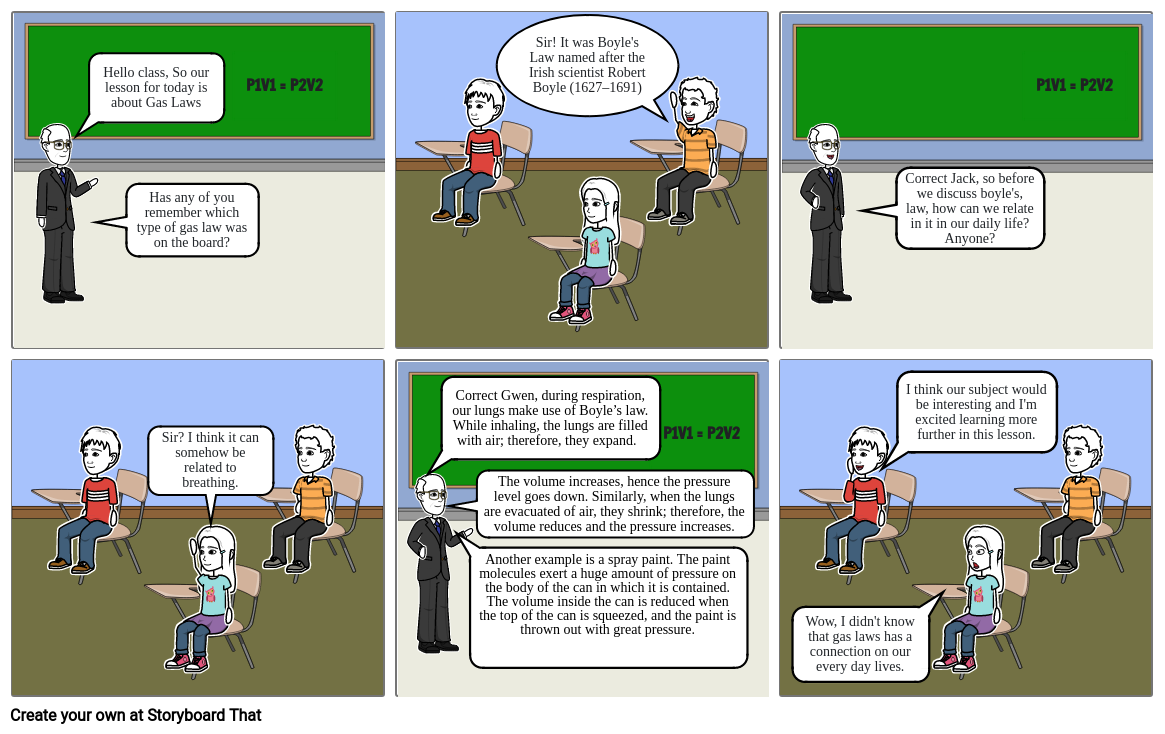
- Hello class, So our lesson for today is about Gas Laws
- Has any of you remember which type of gas law was on the board?
- P1V1 = P2V2
- Sir! It was Boyle's Law named after the Irish scientist Robert Boyle (1627–1691)
- Correct Jack, so before we discuss boyle's, law, how can we relate in it in our daily life? Anyone?
- P1V1 = P2V2
- Sir? I think it can somehow be related to breathing.
- Correct Gwen, during respiration, our lungs make use of Boyle’s law. While inhaling, the lungs are filled with air; therefore, they expand.
- The volume increases, hence the pressure level goes down. Similarly, when the lungs are evacuated of air, they shrink; therefore, the volume reduces and the pressure increases.
- Another example is a spray paint. The paint molecules exert a huge amount of pressure on the body of the can in which it is contained. The volume inside the can is reduced when the top of the can is squeezed, and the paint is thrown out with great pressure.
- P1V1 = P2V2
- Wow, I didn't know that gas laws has a connection on our every day lives.
- I think our subject would be interesting and I'm excited learning more further in this lesson.
Ustvarjenih več kot 30 milijonov snemalnih knjig

