Science Atomic theory Pt.2
Snemalna Knjiga Besedilo
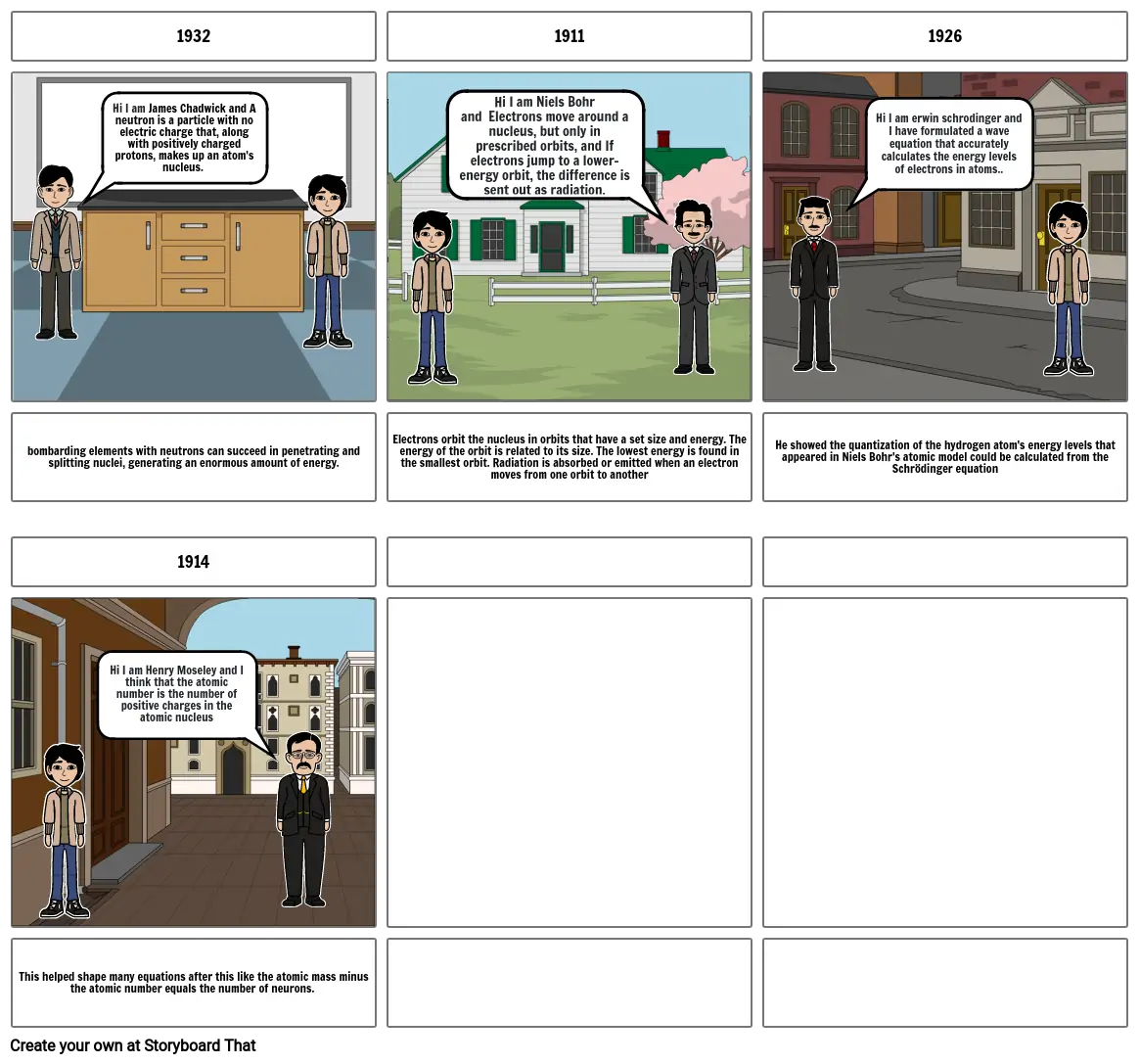
- 1932
- Hi I am James Chadwick and A neutron is a particle with no electric charge that, along with positively charged protons, makes up an atom's nucleus.
- 1911
- Hi I am Niels Bohr and Electrons move around a nucleus, but only in prescribed orbits, and If electrons jump to a lower-energy orbit, the difference is sent out as radiation.
- 1926
- Hi I am erwin schrodinger and I have formulated a wave equation that accurately calculates the energy levels of electrons in atoms..
- bombarding elements with neutrons can succeed in penetrating and splitting nuclei, generating an enormous amount of energy.
- 1914
- Hi I am Henry Moseley and I think that the atomic number is the number of positive charges in the atomic nucleus
- Electrons orbit the nucleus in orbits that have a set size and energy. The energy of the orbit is related to its size. The lowest energy is found in the smallest orbit. Radiation is absorbed or emitted when an electron moves from one orbit to another
- He showed the quantization of the hydrogen atom's energy levels that appeared in Niels Bohr's atomic model could be calculated from the Schrödinger equation
- This helped shape many equations after this like the atomic mass minus the atomic number equals the number of neurons.
Ustvarjenih več kot 30 milijonov snemalnih knjig
Brez Prenosov, Brez Kreditne Kartice in Brez Prijave!


