Chem Project
Snemalna Knjiga Besedilo
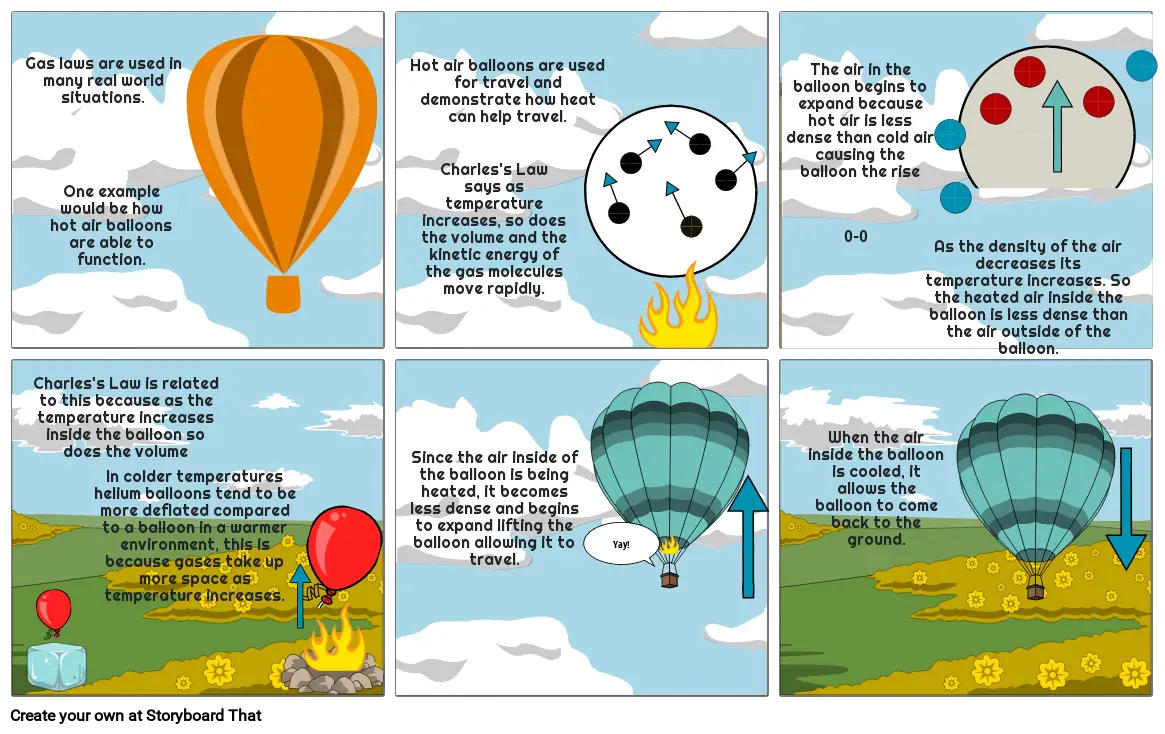
- Gas laws are used in many real world situations.
- One example would be how hot air balloons are able to function.
- Hot air balloons are used for travel and demonstrate how heat can help travel.
- Charles's Law says as temperature increases, so does the volume and the kinetic energy of the gas molecules move rapidly.
- The air in the balloon begins to expand because hot air is less dense than cold air causing the balloon the rise
- 0-0
- As the density of the air decreases its temperature increases. So the heated air inside the balloon is less dense than the air outside of the balloon.
- Charles's Law is related to this because as the temperature increases inside the balloon so does the volume
- In colder temperatures helium balloons tend to be more deflated compared to a balloon in a warmer environment, this is because gases take up more space as temperature increases.
- Since the air inside of the balloon is being heated, it becomes less dense and begins to expand lifting the balloon allowing it to travel.
- Yay!
- When the air inside the balloon is cooled, it allows the balloon to come back to the ground.
Ustvarjenih več kot 30 milijonov snemalnih knjig

