chemistry
Text z Príbehu
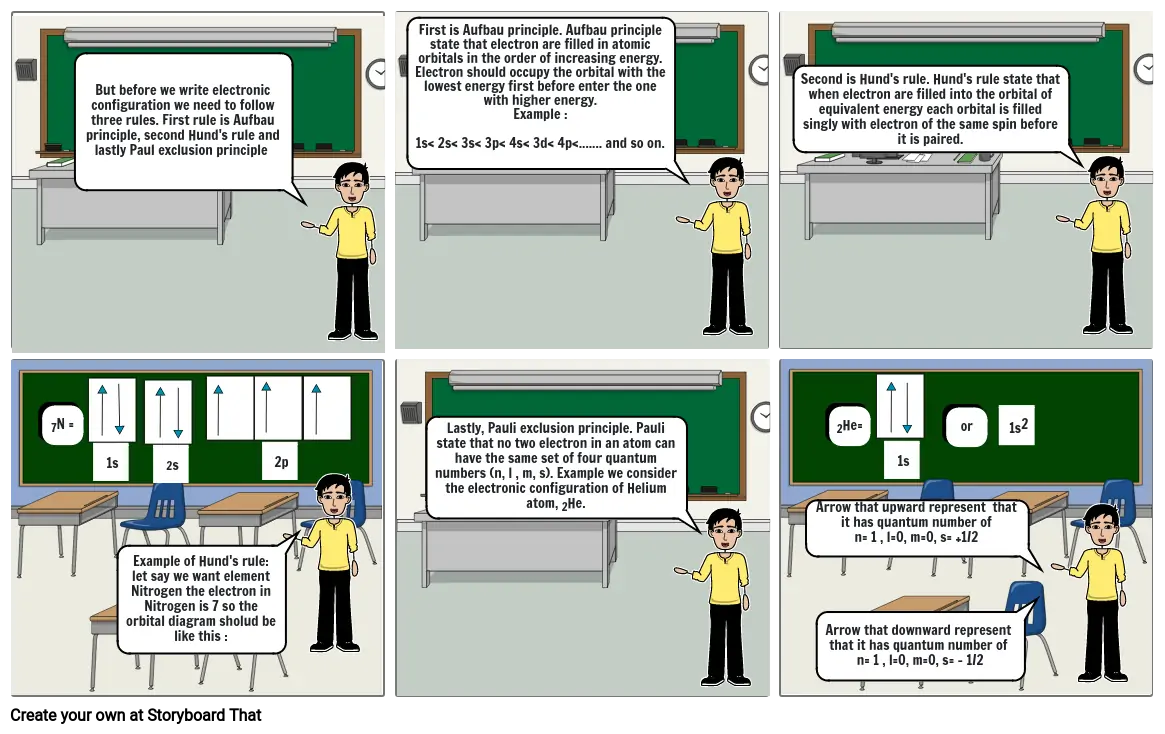
- But before we write electronic configuration we need to follow three rules. First rule is Aufbau principle, second Hund's rule and lastly Paul exclusion principle
- First is Aufbau principle. Aufbau principle state that electron are filled in atomic orbitals in the order of increasing energy. Electron should occupy the orbital with the lowest energy first before enter the one with higher energy. Example :1s< 2s< 3s< 3p< 4s< 3d< 4p<....... and so on.
- Second is Hund's rule. Hund's rule state that when electron are filled into the orbital of equivalent energy each orbital is filled singly with electron of the same spin before it is paired.
- 7N =
- 1s
- Example of Hund's rule:let say we want element Nitrogen the electron in Nitrogen is 7 so the orbital diagram sholud be like this :
- 2s
- 2p
- Lastly, Pauli exclusion principle. Pauli state that no two electron in an atom can have the same set of four quantum numbers (n, l , m, s). Example we consider the electronic configuration of Helium atom, 2He.
- Arrow that upward represent that it has quantum number ofn= 1 , l=0, m=0, s= +1/2
- Arrow that downward represent that it has quantum number of n= 1 , l=0, m=0, s= - 1/2
- 2He=
- 1s
- or
- 1s2
Bolo vytvorených viac ako 30 miliónov storyboardov

