Mrs. Glaze on Chemical and Physical Changes of Matter.
Text z Príbehu
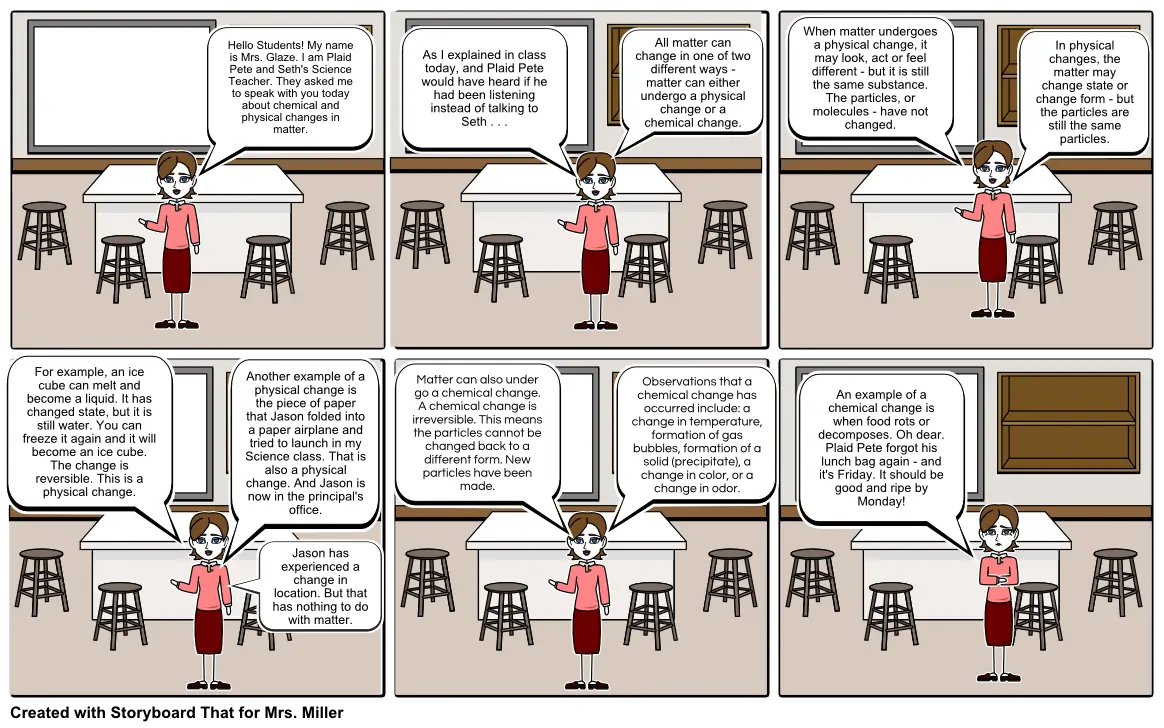
- Hello Students! My name is Mrs. Glaze. I am Plaid Pete and Seth's Science Teacher. They asked me to speak with you today about chemical and physical changes in matter.
- As I explained in class today, and Plaid Pete would have heard if he had been listening instead of talking to Seth . . .
- All matter can change in one of two different ways - matter can either undergo a physical change or a chemical change.
- When matter undergoes a physical change, it may look, act or feel different - but it is still the same substance. The particles, or molecules - have not changed.
- In physical changes, the matter may change state or change form - but the particles are still the same particles.
- For example, an ice cube can melt and become a liquid. It has changed state, but it is still water. You can freeze it again and it will become an ice cube. The change is reversible. This is a physical change.
- Another example of a physical change is the piece of paper that Jason folded into a paper airplane and tried to launch in my Science class. That is also a physical change. And Jason is now in the principal's office.
- Jason has experienced a change in location. But that has nothing to do with matter.
- Matter can also under go a chemical change. A chemical change is irreversible. This means the particles cannot be changed back to a different form. New particles have been made.
- Observations that a chemical change has occurred include: a change in temperature, formation of gas bubbles, formation of a solid (precipitate), a change in color, or a change in odor.
- An example of a chemical change is when food rots or decomposes. Oh dear. Plaid Pete forgot his lunch bag again - and it's Friday. It should be good and ripe by Monday!
Bolo vytvorených viac ako 30 miliónov storyboardov

