Science Comic Strip
Storyboard Popis
Tim Myers Pd. 7
Text z Príbehu
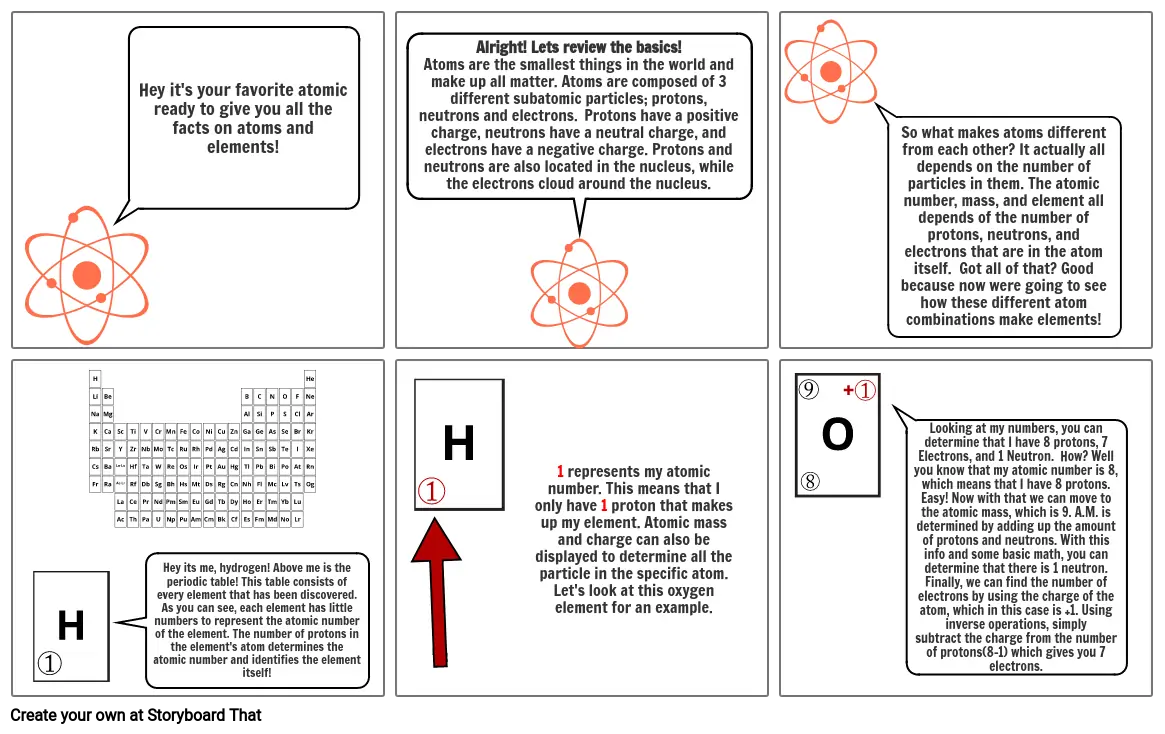
- Hey it's your favorite atomic ready to give you all the facts on atoms and elements!
- Alright! Lets review the basics!Atoms are the smallest things in the world and make up all matter. Atoms are composed of 3 different subatomic particles; protons, neutrons and electrons. Protons have a positive charge, neutrons have a neutral charge, and electrons have a negative charge. Protons and neutrons are also located in the nucleus, while the electrons cloud around the nucleus.
- So what makes atoms different from each other? It actually all depends on the number of particles in them. The atomic number, mass, and element all depends of the number of protons, neutrons, and electrons that are in the atom itself. Got all of that? Good because now were going to see how these different atom combinations make elements!
- Hey its me, hydrogen! Above me is the periodic table! This table consists of every element that has been discovered. As you can see, each element has little numbers to represent the atomic number of the element. The number of protons in the element's atom determines the atomic number and identifies the element itself!
- 1 represents my atomic number. This means that I only have 1 proton that makes up my element. Atomic mass and charge can also be displayed to determine all the particle in the specific atom. Let's look at this oxygen element for an example.
- Looking at my numbers, you can determine that I have 8 protons, 7 Electrons, and 1 Neutron. How? Well you know that my atomic number is 8, which means that I have 8 protons. Easy! Now with that we can move to the atomic mass, which is 9. A.M. is determined by adding up the amount of protons and neutrons. With this info and some basic math, you can determine that there is 1 neutron. Finally, we can find the number of electrons by using the charge of the atom, which in this case is +1. Using inverse operations, simply subtract the charge from the number of protons(8-1) which gives you 7 electrons.
Bolo vytvorených viac ako 30 miliónov storyboardov

