Combination Of Solids
Text z Príbehu
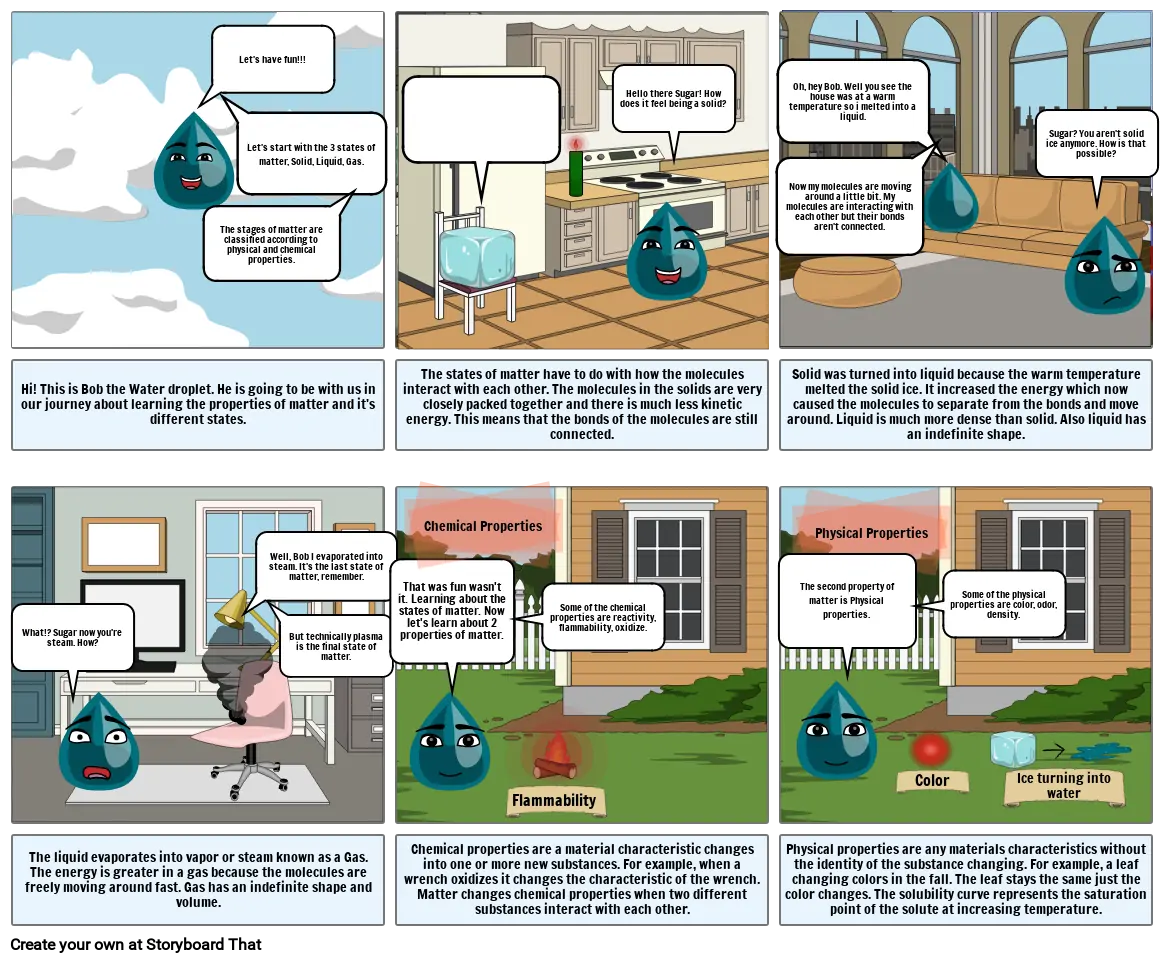
- Let's have fun!!!
- The stages of matter are classified according to physical and chemical properties.
- Let's start with the 3 states of matter, Solid, Liquid, Gas.
- Hey Bob! It feels nice being a solid. Did you know that when you're a solid the molecules are very close together and barely move.
- Hello there Sugar! How does it feel being a solid?
- Now my molecules are moving around a little bit. My molecules are interacting with each other but their bonds aren't connected.
- Oh, hey Bob. Well you see the house was at a warm temperature so i melted into a liquid.
- Sugar? You aren't solid ice anymore. How is that possible?
- What!? Sugar now you're steam. How?
- Hi! This is Bob the Water droplet. He is going to be with us in our journey about learning the properties of matter and it's different states.
- Well, Bob I evaporated into steam. It's the last state of matter, remember.
- But technically plasma is the final state of matter.
- That was fun wasn't it. Learning about the states of matter. Now let's learn about 2 properties of matter.
- The states of matter have to do with how the molecules interact with each other. The molecules in the solids are very closely packed together and there is much less kinetic energy. This means that the bonds of the molecules are still connected.
- Chemical Properties
- Some of the chemical properties are reactivity, flammability, oxidize.
- The second property of matter is Physical properties.
- Solid was turned into liquid because the warm temperature melted the solid ice. It increased the energy which now caused the molecules to separate from the bonds and move around. Liquid is much more dense than solid. Also liquid has an indefinite shape.
- Physical Properties
- Some of the physical properties are color, odor, density.
- The liquid evaporates into vapor or steam known as a Gas. The energy is greater in a gas because the molecules are freely moving around fast. Gas has an indefinite shape and volume.
- Chemical properties are a material characteristic changes into one or more new substances. For example, when a wrench oxidizes it changes the characteristic of the wrench. Matter changes chemical properties when two different substances interact with each other.
- Flammability
- Physical properties are any materials characteristics without the identity of the substance changing. For example, a leaf changing colors in the fall. The leaf stays the same just the color changes. The solubility curve represents the saturation point of the solute at increasing temperature.
- Color
- Ice turning into water
Bolo vytvorených viac ako 30 miliónov storyboardov

