Development of the Atomic Theory Comic Part 2
Text z Príbehu
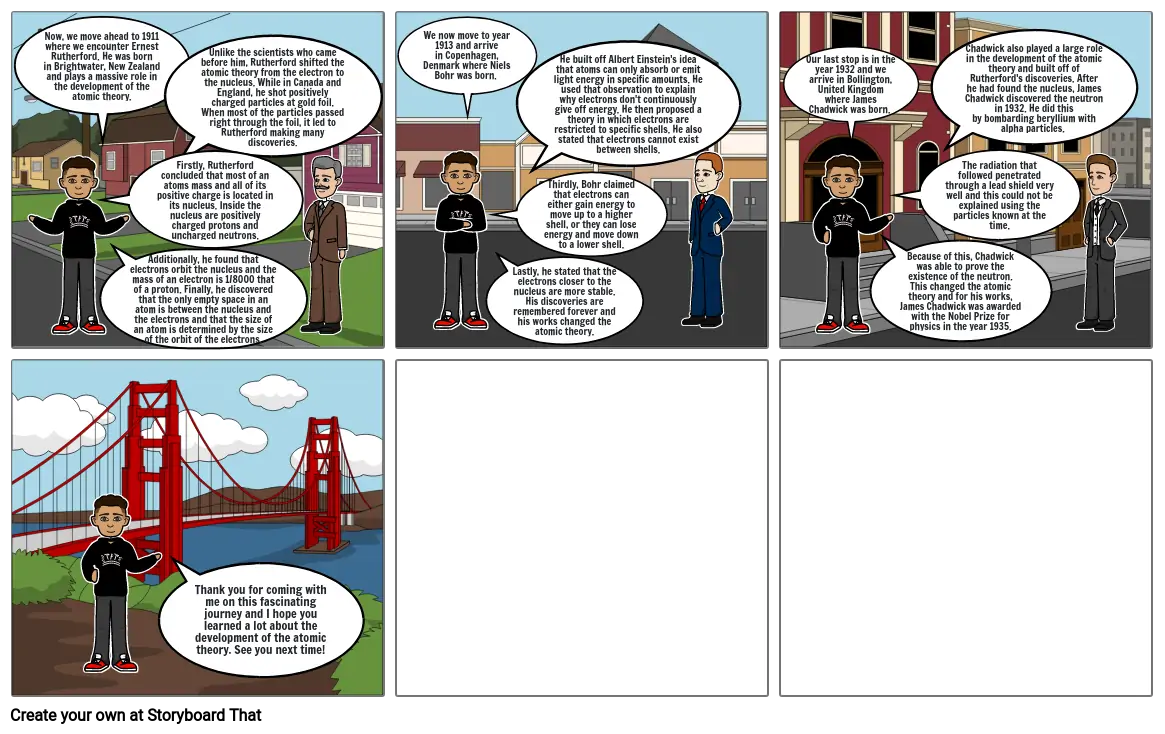
- Now, we move ahead to 1911 where we encounter Ernest Rutherford. He was born in Brightwater, New Zealand and plays a massive role in the development of the atomic theory.
- Firstly, Rutherford concluded that most of an atoms mass and all of its positive charge is located in its nucleus. Inside the nucleus are positively charged protons and uncharged neutrons.
- Additionally, he found that electrons orbit the nucleus and the mass of an electron is 1/8000 that of a proton. Finally, he discovered that the only empty space in an atom is between the nucleus and the electrons and that the size of an atom is determined by the size of the orbit of the electrons.
- Unlike the scientists who came before him, Rutherford shifted the atomic theory from the electron to the nucleus. While in Canada and England, he shot positively charged particles at gold foil. When most of the particles passed right through the foil, it led to Rutherford making many discoveries.
- We now move to year 1913 and arrive in Copenhagen, Denmark where Niels Bohr was born.
- Lastly, he stated that the electrons closer to the nucleus are more stable. His discoveries are remembered forever and his works changed the atomic theory.
- Thirdly, Bohr claimed that electrons can either gain energy to move up to a higher shell, or they can lose energy and move down to a lower shell.
- He built off Albert Einstein's idea that atoms can only absorb or emit light energy in specific amounts. He used that observation to explain why electrons don't continuously give off energy. He then proposed a theory in which electrons are restricted to specific shells. He also stated that electrons cannot exist between shells.
- Our last stop is in the year 1932 and we arrive in Bollington, United Kingdom where James Chadwick was born.
- Because of this, Chadwick was able to prove the existence of the neutron. This changed the atomic theory and for his works, James Chadwick was awarded with the Nobel Prize for physics in the year 1935.
- The radiation that followed penetrated through a lead shield very well and this could not be explained using the particles known at the time.
- Chadwick also played a large role in the development of the atomic theory and built off of Rutherford's discoveries. After he had found the nucleus, James Chadwick discovered the neutron in 1932. He did this by bombarding beryllium with alpha particles.
- Thank you for coming with me on this fascinating journey and I hope you learned a lot about the development of the atomic theory. See you next time!
Bolo vytvorených viac ako 30 miliónov storyboardov

