IKATAN KIMIA
Text z Príbehu
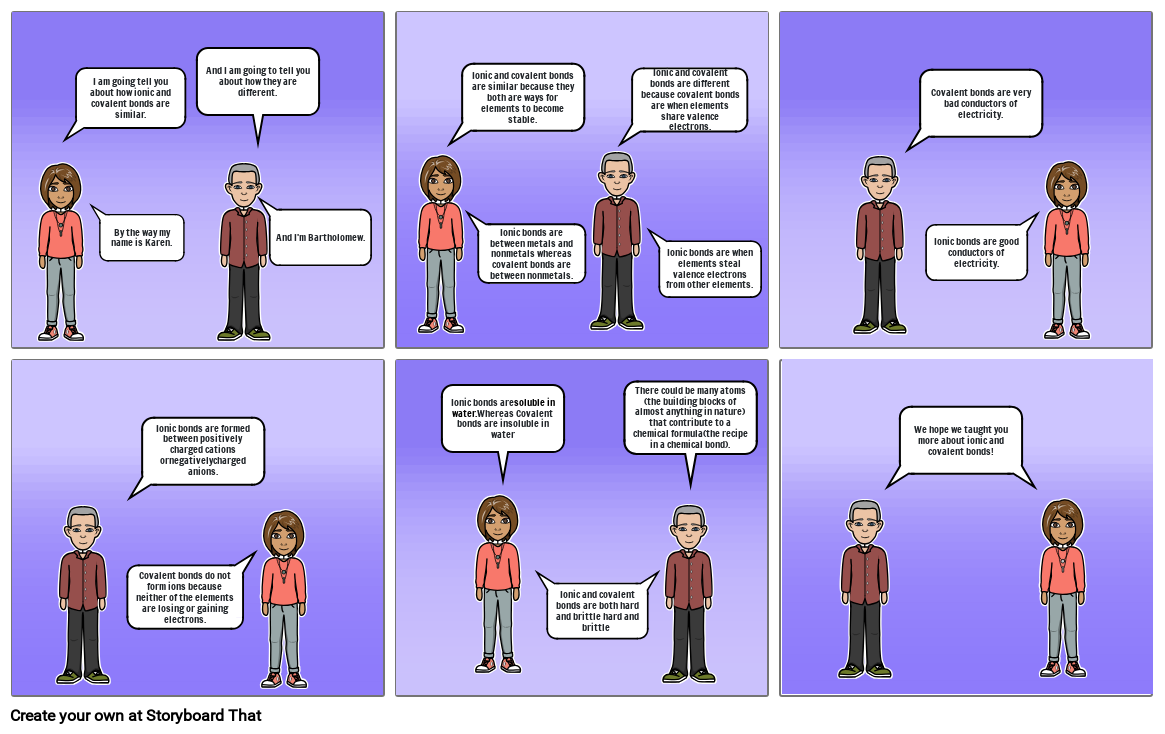
- Šmykľavka: 1
- And I am going to tell you about how they are different.
- I am going tell you about how ionic and covalent bonds are similar.
- And I'm Bartholomew.
- By the way my name is Karen.
- Šmykľavka: 2
- Ionic and covalent bonds are similar because they both are ways for elements to become stable.
- Ionic bonds are between metals and nonmetals whereas covalent bonds are between nonmetals.
- Ionic bonds are when elements steal valence electrons from other elements.
- Šmykľavka: 3
- Covalent bonds are very bad conductors of electricity.
- Šmykľavka: 4
- Ionic bonds are formed between positively charged cations ornegativelycharged anions.
- Covalent bonds do not form ions because neither of the elements are losing or gaining electrons.
- Šmykľavka: 5
- There could be many atoms (the building blocks of almost anything in nature) that contribute to a chemical formula(the recipe in a chemical bond).
- Ionic bonds aresoluble in water.Whereas Covalent bonds are insoluble in water
- Šmykľavka: 6
- We hope we taught you more about ionic and covalent bonds!
- Šmykľavka: 0
- Ionic and covalent bonds are different because covalent bonds are when elements share valence electrons.
- Ionic bonds are good conductors of electricity.
- Ionic and covalent bonds are both hard and brittle hard and brittle
Bolo vytvorených viac ako 30 miliónov storyboardov

