Acid rain is a growing environmental problem in recent times. The main cause of acid rain is pollution from motor vehicles or factories.
One of the industrial exhaust gases is sulfur dioxide (SO2), reacting with water in the air can be written:
SO2(g) + H2O(l) → H2SO3(aq)
SO3(g) + H2O(l) → H2SO4(aq)
Other pollutants is nitrogen dioxide (NO2) which is produced from the reaction of N2 and O2 in coal combustion.
This NO2 compound when dissolved in water, it will form HNO3 and NO2 gas. Acid rain will have a negative impact on plants, namely inhibiting germination and reproduction which will directly poison the delicate shoots to the roots.
For aquatic organisms, acid rain will inhibit the metabolism of aquatic biota. In addition, acid rain also damages buildings. Its sulfate content is corrosive to iron building materials. Meanwhile, for humans, acid rain causes itching, respiratory problems, and others.
Acid solutions and alkaline solutions are electrolyte solutions (can conduct electricity). Characteristics, acid has a sour taste. For example, kitchen vinegar has a sour taste because it contains acetic acid.
Vitamin C, it also tastes sour because it contains ascorbic acid. Lime fruit also has a sour taste because it contains citric acid.
Detergent
Oo, Chemistry class turns out to be.. hehehe.. I bought a base at the shop. to wash clothes.
Bases have a bitter taste and are slippery when touched. For example, whiting has a bitter taste and soap when touched feels slippery.
Yes, I'm home. Assalamualaikum...
Acid rain is a growing environmental problem in recent times. The main cause of acid rain is pollution from motor vehicles or factories.
One of the industrial exhaust gases is sulfur dioxide (SO2), reacting with water in the air can be written:
SO2(g) + H2O(l) → H2SO3(aq)
SO3(g) + H2O(l) → H2SO4(aq)
Other pollutants is nitrogen dioxide (NO2) which is produced from the reaction of N2 and O2 in coal combustion.
This NO2 compound when dissolved in water, it will form HNO3 and NO2 gas. Acid rain will have a negative impact on plants, namely inhibiting germination and reproduction which will directly poison the delicate shoots to the roots.
For aquatic organisms, acid rain will inhibit the metabolism of aquatic biota. In addition, acid rain also damages buildings. Its sulfate content is corrosive to iron building materials. Meanwhile, for humans, acid rain causes itching, respiratory problems, and others.
Acid solutions and alkaline solutions are electrolyte solutions (can conduct electricity). Characteristics, acid has a sour taste. For example, kitchen vinegar has a sour taste because it contains acetic acid.
Vitamin C, it also tastes sour because it contains ascorbic acid. Lime fruit also has a sour taste because it contains citric acid.
Detergent
Oo, Chemistry class turns out to be.. hehehe.. I bought a base at the shop. to wash clothes.
Bases have a bitter taste and are slippery when touched. For example, whiting has a bitter taste and soap when touched feels slippery.
Yes, I'm home. Assalamualaikum...
Acid rain is a growing environmental problem in recent times. The main cause of acid rain is pollution from motor vehicles or factories.
One of the industrial exhaust gases is sulfur dioxide (SO2), reacting with water in the air can be written:
SO2(g) + H2O(l) → H2SO3(aq)
SO3(g) + H2O(l) → H2SO4(aq)
Other pollutants is nitrogen dioxide (NO2) which is produced from the reaction of N2 and O2 in coal combustion.
This NO2 compound when dissolved in water, it will form HNO3 and NO2 gas. Acid rain will have a negative impact on plants, namely inhibiting germination and reproduction which will directly poison the delicate shoots to the roots.
For aquatic organisms, acid rain will inhibit the metabolism of aquatic biota. In addition, acid rain also damages buildings. Its sulfate content is corrosive to iron building materials. Meanwhile, for humans, acid rain causes itching, respiratory problems, and others.
Acid solutions and alkaline solutions are electrolyte solutions (can conduct electricity). Characteristics, acid has a sour taste. For example, kitchen vinegar has a sour taste because it contains acetic acid.
Vitamin C, it also tastes sour because it contains ascorbic acid. Lime fruit also has a sour taste because it contains citric acid.
Detergent
Oo, Chemistry class turns out to be.. hehehe.. I bought a base at the shop. to wash clothes.
Bases have a bitter taste and are slippery when touched. For example, whiting has a bitter taste and soap when touched feels slippery.
Yes, I'm home. Assalamualaikum...
Acid rain is a growing environmental problem in recent times. The main cause of acid rain is pollution from motor vehicles or factories.
One of the industrial exhaust gases is sulfur dioxide (SO2), reacting with water in the air can be written:
SO2(g) + H2O(l) → H2SO3(aq)
SO3(g) + H2O(l) → H2SO4(aq)
Other pollutants is nitrogen dioxide (NO2) which is produced from the reaction of N2 and O2 in coal combustion.
This NO2 compound when dissolved in water, it will form HNO3 and NO2 gas. Acid rain will have a negative impact on plants, namely inhibiting germination and reproduction which will directly poison the delicate shoots to the roots.
For aquatic organisms, acid rain will inhibit the metabolism of aquatic biota. In addition, acid rain also damages buildings. Its sulfate content is corrosive to iron building materials. Meanwhile, for humans, acid rain causes itching, respiratory problems, and others.
Acid solutions and alkaline solutions are electrolyte solutions (can conduct electricity). Characteristics, acid has a sour taste. For example, kitchen vinegar has a sour taste because it contains acetic acid.
Vitamin C, it also tastes sour because it contains ascorbic acid. Lime fruit also has a sour taste because it contains citric acid.
Detergent
Oo, Chemistry class turns out to be.. hehehe.. I bought a base at the shop. to wash clothes.
Bases have a bitter taste and are slippery when touched. For example, whiting has a bitter taste and soap when touched feels slippery.
Yes, I'm home. Assalamualaikum...
Acid rain is a growing environmental problem in recent times. The main cause of acid rain is pollution from motor vehicles or factories.
One of the industrial exhaust gases is sulfur dioxide (SO2), reacting with water in the air can be written:
SO2(g) + H2O(l) → H2SO3(aq)
SO3(g) + H2O(l) → H2SO4(aq)
Other pollutants is nitrogen dioxide (NO2) which is produced from the reaction of N2 and O2 in coal combustion.
This NO2 compound when dissolved in water, it will form HNO3 and NO2 gas. Acid rain will have a negative impact on plants, namely inhibiting germination and reproduction which will directly poison the delicate shoots to the roots.
For aquatic organisms, acid rain will inhibit the metabolism of aquatic biota. In addition, acid rain also damages buildings. Its sulfate content is corrosive to iron building materials. Meanwhile, for humans, acid rain causes itching, respiratory problems, and others.
Acid solutions and alkaline solutions are electrolyte solutions (can conduct electricity). Characteristics, acid has a sour taste. For example, kitchen vinegar has a sour taste because it contains acetic acid.
Vitamin C, it also tastes sour because it contains ascorbic acid. Lime fruit also has a sour taste because it contains citric acid.
Detergent
Oo, Chemistry class turns out to be.. hehehe.. I bought a base at the shop. to wash clothes.
Bases have a bitter taste and are slippery when touched. For example, whiting has a bitter taste and soap when touched feels slippery.
Yes, I'm home. Assalamualaikum...
Acid rain is a growing environmental problem in recent times. The main cause of acid rain is pollution from motor vehicles or factories.
One of the industrial exhaust gases is sulfur dioxide (SO2), reacting with water in the air can be written:
SO2(g) + H2O(l) → H2SO3(aq)
SO3(g) + H2O(l) → H2SO4(aq)
Other pollutants is nitrogen dioxide (NO2) which is produced from the reaction of N2 and O2 in coal combustion.
This NO2 compound when dissolved in water, it will form HNO3 and NO2 gas. Acid rain will have a negative impact on plants, namely inhibiting germination and reproduction which will directly poison the delicate shoots to the roots.
For aquatic organisms, acid rain will inhibit the metabolism of aquatic biota. In addition, acid rain also damages buildings. Its sulfate content is corrosive to iron building materials. Meanwhile, for humans, acid rain causes itching, respiratory problems, and others.
Acid solutions and alkaline solutions are electrolyte solutions (can conduct electricity). Characteristics, acid has a sour taste. For example, kitchen vinegar has a sour taste because it contains acetic acid.
Vitamin C, it also tastes sour because it contains ascorbic acid. Lime fruit also has a sour taste because it contains citric acid.
Detergent
Oo, Chemistry class turns out to be.. hehehe.. I bought a base at the shop. to wash clothes.
Bases have a bitter taste and are slippery when touched. For example, whiting has a bitter taste and soap when touched feels slippery.
Yes, I'm home. Assalamualaikum...
Acid rain is a growing environmental problem in recent times. The main cause of acid rain is pollution from motor vehicles or factories.
One of the industrial exhaust gases is sulfur dioxide (SO2), reacting with water in the air can be written:
SO2(g) + H2O(l) → H2SO3(aq)
SO3(g) + H2O(l) → H2SO4(aq)
Other pollutants is nitrogen dioxide (NO2) which is produced from the reaction of N2 and O2 in coal combustion.
This NO2 compound when dissolved in water, it will form HNO3 and NO2 gas. Acid rain will have a negative impact on plants, namely inhibiting germination and reproduction which will directly poison the delicate shoots to the roots.
For aquatic organisms, acid rain will inhibit the metabolism of aquatic biota. In addition, acid rain also damages buildings. Its sulfate content is corrosive to iron building materials. Meanwhile, for humans, acid rain causes itching, respiratory problems, and others.
Acid solutions and alkaline solutions are electrolyte solutions (can conduct electricity). Characteristics, acid has a sour taste. For example, kitchen vinegar has a sour taste because it contains acetic acid.
Vitamin C, it also tastes sour because it contains ascorbic acid. Lime fruit also has a sour taste because it contains citric acid.
Detergent
Oo, Chemistry class turns out to be.. hehehe.. I bought a base at the shop. to wash clothes.
Bases have a bitter taste and are slippery when touched. For example, whiting has a bitter taste and soap when touched feels slippery.
Yes, I'm home. Assalamualaikum...
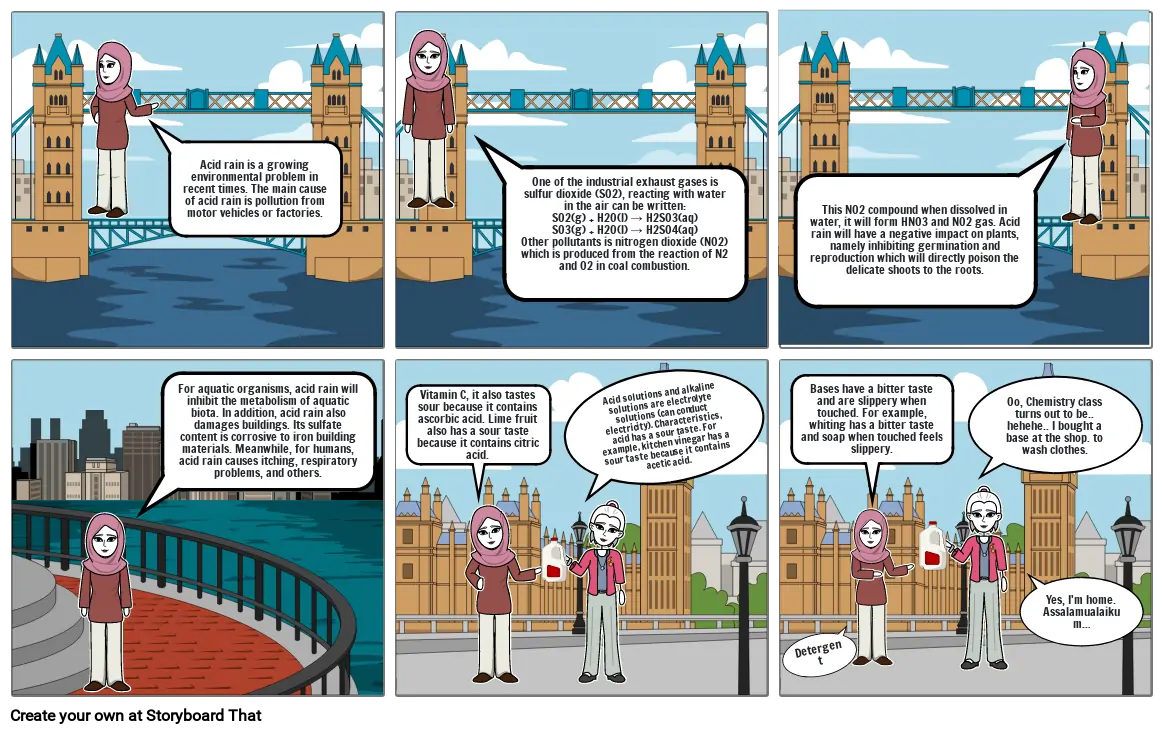
Text z Príbehu
- Acid rain is a growing environmental problem in recent times. The main cause of acid rain is pollution from motor vehicles or factories.
- One of the industrial exhaust gases is sulfur dioxide (SO2), reacting with water in the air can be written: SO2(g) + H2O(l) → H2SO3(aq)SO3(g) + H2O(l) → H2SO4(aq)Other pollutants is nitrogen dioxide (NO2) which is produced from the reaction of N2 and O2 in coal combustion.
- This NO2 compound when dissolved in water, it will form HNO3 and NO2 gas. Acid rain will have a negative impact on plants, namely inhibiting germination and reproduction which will directly poison the delicate shoots to the roots.
- For aquatic organisms, acid rain will inhibit the metabolism of aquatic biota. In addition, acid rain also damages buildings. Its sulfate content is corrosive to iron building materials. Meanwhile, for humans, acid rain causes itching, respiratory problems, and others.
- Vitamin C, it also tastes sour because it contains ascorbic acid. Lime fruit also has a sour taste because it contains citric acid.
- Acid solutions and alkaline solutions are electrolyte solutions (can conduct electricity). Characteristics, acid has a sour taste. For example, kitchen vinegar has a sour taste because it contains acetic acid.
- Detergent
- Bases have a bitter taste and are slippery when touched. For example, whiting has a bitter taste and soap when touched feels slippery.
- Oo, Chemistry class turns out to be.. hehehe.. I bought a base at the shop. to wash clothes.
- Yes, I'm home. Assalamualaikum...


