Unknown Story
Text z Príbehu
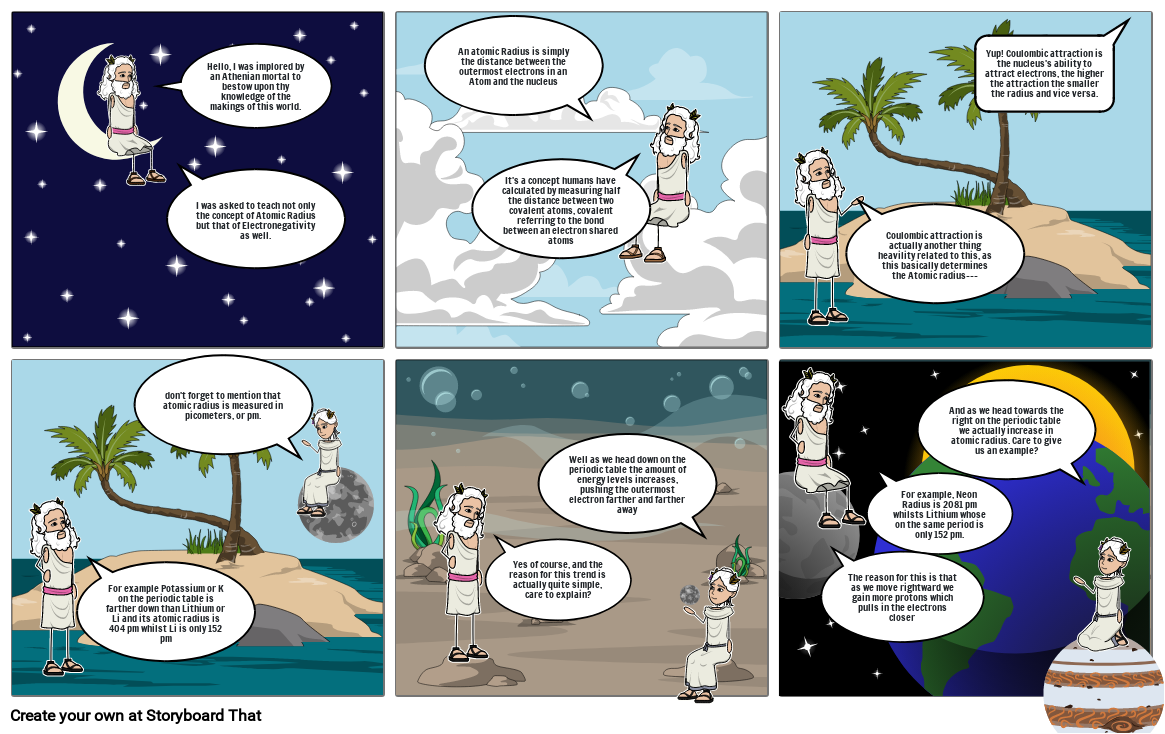
- Hello, I was implored by an Athenian mortal to bestow upon thy knowledge of the makings of this world.
- I was asked to teach not only the concept of Atomic Radius but that of Electronegativity as well.
- An atomic Radius is simply the distance between the outermost electrons in an Atom and the nucleus
- It's a concept humans have calculated by measuring half the distance between two covalent atoms, covalent referring to the bond between an electron shared atoms
- Coulombic attraction is actually another thing heavility related to this, as this basically determines the Atomic radius---
- Yup! Coulombic attraction is the nucleus's ability to attract electrons, the higher the attraction the smaller the radius and vice versa.
- For example Potassium or K on the periodic table is farther down than Lithium or Li and its atomic radius is 404 pm whilst Li is only 152 pm
- don't forget to mention that atomic radius is measured in picometers, or pm.
- Yes of course, and the reason for this trend is actually quite simple, care to explain?
- Well as we head down on the periodic table the amount of energy levels increases, pushing the outermost electron farther and farther away
- The reason for this is that as we move rightward we gain more protons which pulls in the electrons closer
- For example, Neon Radius is 2081 pm whilsts Lithium whose on the same period is only 152 pm.
- And as we head towards the right on the periodic table we actually increase in atomic radius. Care to give us an example?
Bolo vytvorených viac ako 30 miliónov storyboardov

