Discovered the Neutron
Text z Príbehu
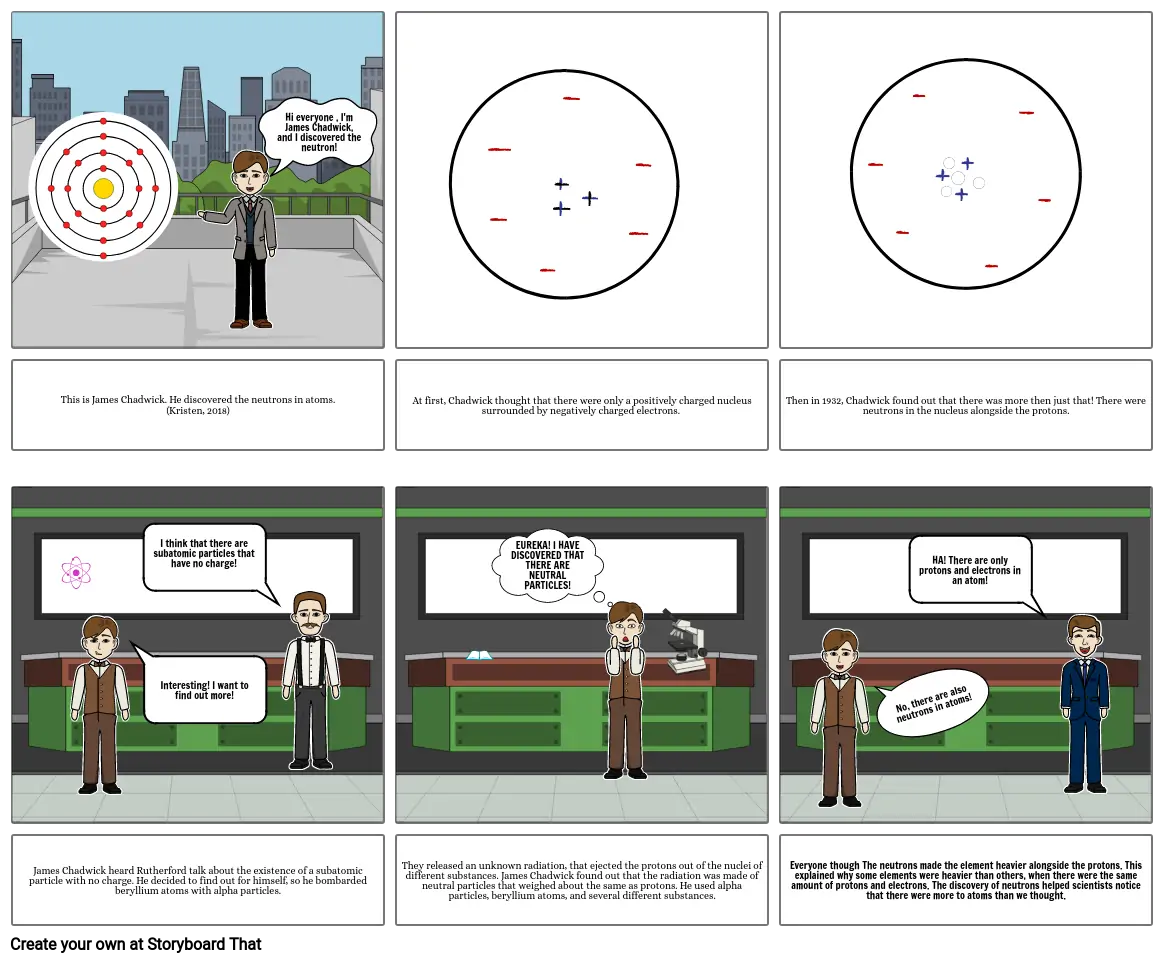
- Hi everyone , I'm James Chadwick, and I discovered the neutron!
- This is James Chadwick. He discovered the neutrons in atoms. (Kristen, 2018)
- Interesting! I want to find out more!
- I think that there are subatomic particles that have no charge!
- At first, Chadwick thought that there were only a positively charged nucleus surrounded by negatively charged electrons.
- EUREKA! I HAVE DISCOVERED THAT THERE ARE NEUTRAL PARTICLES!
- Then in 1932, Chadwick found out that there was more then just that! There were neutrons in the nucleus alongside the protons.
- No, there are also neutrons in atoms!
- HA! There are only protons and electrons in an atom!
- James Chadwick heard Rutherford talk about the existence of a subatomic particle with no charge. He decided to find out for himself, so he bombarded beryllium atoms with alpha particles.
- They released an unknown radiation, that ejected the protons out of the nuclei of different substances. James Chadwick found out that the radiation was made of neutral particles that weighed about the same as protons. He used alpha particles, beryllium atoms, and several different substances.
- Everyone though The neutrons made the element heavier alongside the protons. This explained why some elements were heavier than others, when there were the same amount of protons and electrons. The discovery of neutrons helped scientists notice that there were more to atoms than we thought.
Bolo vytvorených viac ako 30 miliónov storyboardov

