Ionic Compound Comic
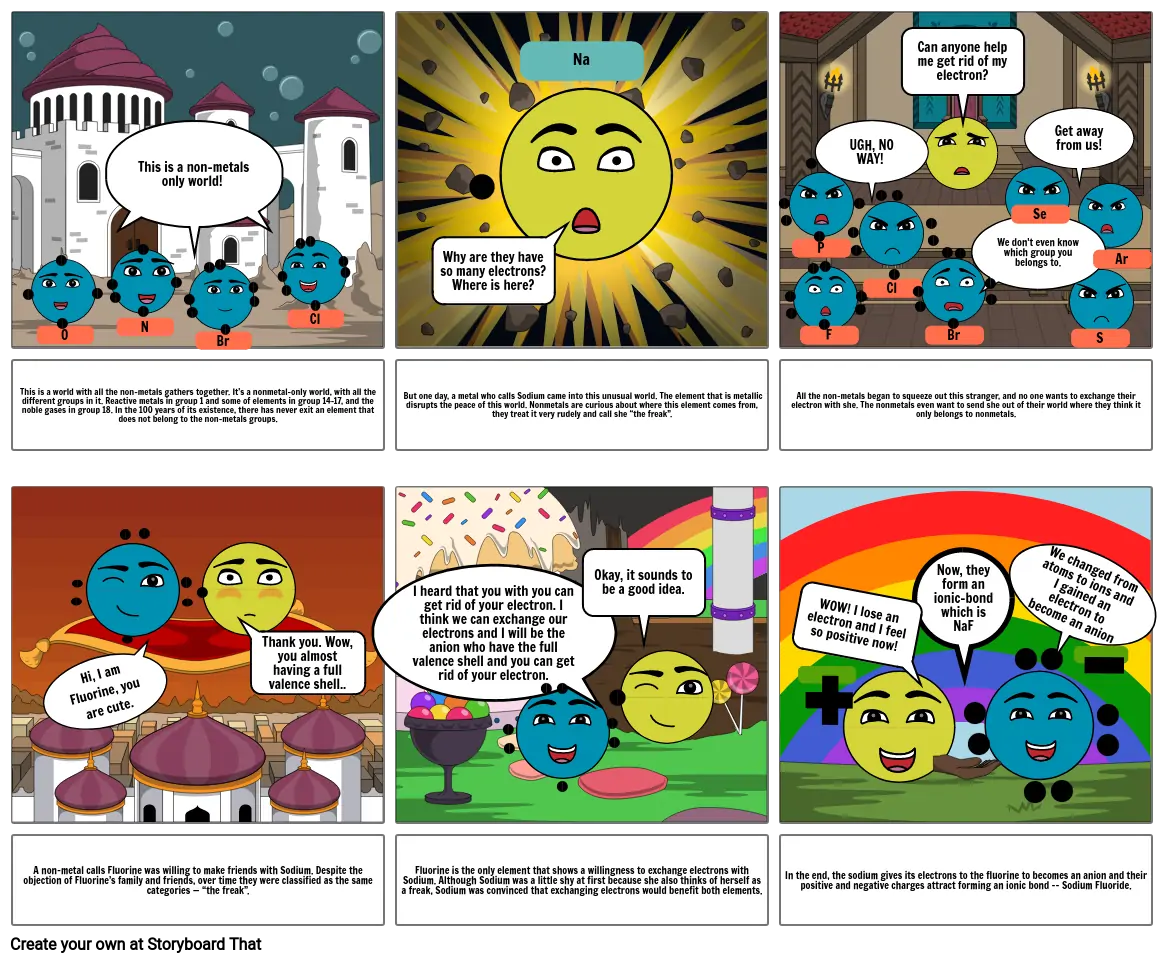
This is a world with all the non-metals gathers together. It's a nonmetal-only world, with all the different groups in it. Reactive metals in group 1 and some of elements in group 14-17, and the noble gases in group 18.In the 100 years of its existence, there has never exit an element that does not belong to the non-metals groups.
But one day, a metal who calls Sodium came into this unusual world.The element that is metallic disrupts the peace of this world.Nonmetals are curious about where this element comes from, they treat it very rudely and call she “the freak”.
All the non-metals began to squeeze out this stranger, and no one wants to exchange their electron with she. The nonmetalseven want to send she out of their world where they think it only belongs to nonmetals.
A non-metal calls Fluorine was willing to make friends with Sodium. Despite the objection of Fluorine’s family and friends, over time they were classified as the same categories — “the freak”.
Fluorine is the only element that shows a willingness to exchange electrons with Sodium. Although Sodium was a little shy at first because she also thinks of herself as a freak, Sodium was convinced that exchanging electrons would benefit both elements.
In the end, the sodium gives its electrons to the fluorine to becomes an anion and their positive and negative charges attract forming an ionic bond -- Sodium Fluoride.
This is a non-metals only world!
O
N
Cl
Br
Why are they have so many electrons? Where is here?
Can anyone help me get rid of my electron?
UGH, NO WAY!
We don't even know which group you belongs to.
Get away from us!
P
F
Cl
Br
Na
S
Ar
Se
Thank you. Wow, you almost having a full valence shell..
I heard that you with you can get rid of your electron. I think we can exchange our electrons and I will be the anion who have the full valence shell and you can get rid of your electron.
Okay, it sounds to be a good idea.
Now, they form an ionic-bond which is NaF
WOW! I lose an electron and I feel so positive now!
+
-
We changed from atoms to ions and I gained an electron to become an anion
This is a world with all the non-metals gathers together. It's a nonmetal-only world, with all the different groups in it. Reactive metals in group 1 and some of elements in group 14-17, and the noble gases in group 18.In the 100 years of its existence, there has never exit an element that does not belong to the non-metals groups.
But one day, a metal who calls Sodium came into this unusual world.The element that is metallic disrupts the peace of this world.Nonmetals are curious about where this element comes from, they treat it very rudely and call she “the freak”.
All the non-metals began to squeeze out this stranger, and no one wants to exchange their electron with she. The nonmetalseven want to send she out of their world where they think it only belongs to nonmetals.
A non-metal calls Fluorine was willing to make friends with Sodium. Despite the objection of Fluorine’s family and friends, over time they were classified as the same categories — “the freak”.
Fluorine is the only element that shows a willingness to exchange electrons with Sodium. Although Sodium was a little shy at first because she also thinks of herself as a freak, Sodium was convinced that exchanging electrons would benefit both elements.
In the end, the sodium gives its electrons to the fluorine to becomes an anion and their positive and negative charges attract forming an ionic bond -- Sodium Fluoride.
This is a non-metals only world!
O
N
Cl
Br
Why are they have so many electrons? Where is here?
Can anyone help me get rid of my electron?
UGH, NO WAY!
We don't even know which group you belongs to.
Get away from us!
P
F
Cl
Br
Na
S
Ar
Se
Thank you. Wow, you almost having a full valence shell..
I heard that you with you can get rid of your electron. I think we can exchange our electrons and I will be the anion who have the full valence shell and you can get rid of your electron.
Okay, it sounds to be a good idea.
Now, they form an ionic-bond which is NaF
WOW! I lose an electron and I feel so positive now!
+
-
We changed from atoms to ions and I gained an electron to become an anion
This is a world with all the non-metals gathers together. It's a nonmetal-only world, with all the different groups in it. Reactive metals in group 1 and some of elements in group 14-17, and the noble gases in group 18.In the 100 years of its existence, there has never exit an element that does not belong to the non-metals groups.
But one day, a metal who calls Sodium came into this unusual world.The element that is metallic disrupts the peace of this world.Nonmetals are curious about where this element comes from, they treat it very rudely and call she “the freak”.
All the non-metals began to squeeze out this stranger, and no one wants to exchange their electron with she. The nonmetalseven want to send she out of their world where they think it only belongs to nonmetals.
A non-metal calls Fluorine was willing to make friends with Sodium. Despite the objection of Fluorine’s family and friends, over time they were classified as the same categories — “the freak”.
Fluorine is the only element that shows a willingness to exchange electrons with Sodium. Although Sodium was a little shy at first because she also thinks of herself as a freak, Sodium was convinced that exchanging electrons would benefit both elements.
In the end, the sodium gives its electrons to the fluorine to becomes an anion and their positive and negative charges attract forming an ionic bond -- Sodium Fluoride.
This is a non-metals only world!
O
N
Cl
Br
Why are they have so many electrons? Where is here?
Can anyone help me get rid of my electron?
UGH, NO WAY!
We don't even know which group you belongs to.
Get away from us!
P
F
Cl
Br
Na
S
Ar
Se
Thank you. Wow, you almost having a full valence shell..
I heard that you with you can get rid of your electron. I think we can exchange our electrons and I will be the anion who have the full valence shell and you can get rid of your electron.
Okay, it sounds to be a good idea.
Now, they form an ionic-bond which is NaF
WOW! I lose an electron and I feel so positive now!
+
-
We changed from atoms to ions and I gained an electron to become an anion
This is a world with all the non-metals gathers together. It's a nonmetal-only world, with all the different groups in it. Reactive metals in group 1 and some of elements in group 14-17, and the noble gases in group 18.In the 100 years of its existence, there has never exit an element that does not belong to the non-metals groups.
But one day, a metal who calls Sodium came into this unusual world.The element that is metallic disrupts the peace of this world.Nonmetals are curious about where this element comes from, they treat it very rudely and call she “the freak”.
All the non-metals began to squeeze out this stranger, and no one wants to exchange their electron with she. The nonmetalseven want to send she out of their world where they think it only belongs to nonmetals.
A non-metal calls Fluorine was willing to make friends with Sodium. Despite the objection of Fluorine’s family and friends, over time they were classified as the same categories — “the freak”.
Fluorine is the only element that shows a willingness to exchange electrons with Sodium. Although Sodium was a little shy at first because she also thinks of herself as a freak, Sodium was convinced that exchanging electrons would benefit both elements.
In the end, the sodium gives its electrons to the fluorine to becomes an anion and their positive and negative charges attract forming an ionic bond -- Sodium Fluoride.
This is a non-metals only world!
O
N
Cl
Br
Why are they have so many electrons? Where is here?
Can anyone help me get rid of my electron?
UGH, NO WAY!
We don't even know which group you belongs to.
Get away from us!
P
F
Cl
Br
Na
S
Ar
Se
Thank you. Wow, you almost having a full valence shell..
I heard that you with you can get rid of your electron. I think we can exchange our electrons and I will be the anion who have the full valence shell and you can get rid of your electron.
Okay, it sounds to be a good idea.
Now, they form an ionic-bond which is NaF
WOW! I lose an electron and I feel so positive now!
+
-
We changed from atoms to ions and I gained an electron to become an anion
This is a world with all the non-metals gathers together. It's a nonmetal-only world, with all the different groups in it. Reactive metals in group 1 and some of elements in group 14-17, and the noble gases in group 18.In the 100 years of its existence, there has never exit an element that does not belong to the non-metals groups.
But one day, a metal who calls Sodium came into this unusual world.The element that is metallic disrupts the peace of this world.Nonmetals are curious about where this element comes from, they treat it very rudely and call she “the freak”.
All the non-metals began to squeeze out this stranger, and no one wants to exchange their electron with she. The nonmetalseven want to send she out of their world where they think it only belongs to nonmetals.
A non-metal calls Fluorine was willing to make friends with Sodium. Despite the objection of Fluorine’s family and friends, over time they were classified as the same categories — “the freak”.
Fluorine is the only element that shows a willingness to exchange electrons with Sodium. Although Sodium was a little shy at first because she also thinks of herself as a freak, Sodium was convinced that exchanging electrons would benefit both elements.
In the end, the sodium gives its electrons to the fluorine to becomes an anion and their positive and negative charges attract forming an ionic bond -- Sodium Fluoride.
This is a non-metals only world!
O
N
Cl
Br
Why are they have so many electrons? Where is here?
Can anyone help me get rid of my electron?
UGH, NO WAY!
We don't even know which group you belongs to.
Get away from us!
P
F
Cl
Br
Na
S
Ar
Se
Thank you. Wow, you almost having a full valence shell..
I heard that you with you can get rid of your electron. I think we can exchange our electrons and I will be the anion who have the full valence shell and you can get rid of your electron.
Okay, it sounds to be a good idea.
Now, they form an ionic-bond which is NaF
WOW! I lose an electron and I feel so positive now!
+
-
We changed from atoms to ions and I gained an electron to become an anion
This is a world with all the non-metals gathers together. It's a nonmetal-only world, with all the different groups in it. Reactive metals in group 1 and some of elements in group 14-17, and the noble gases in group 18.In the 100 years of its existence, there has never exit an element that does not belong to the non-metals groups.
But one day, a metal who calls Sodium came into this unusual world.The element that is metallic disrupts the peace of this world.Nonmetals are curious about where this element comes from, they treat it very rudely and call she “the freak”.
All the non-metals began to squeeze out this stranger, and no one wants to exchange their electron with she. The nonmetalseven want to send she out of their world where they think it only belongs to nonmetals.
A non-metal calls Fluorine was willing to make friends with Sodium. Despite the objection of Fluorine’s family and friends, over time they were classified as the same categories — “the freak”.
Fluorine is the only element that shows a willingness to exchange electrons with Sodium. Although Sodium was a little shy at first because she also thinks of herself as a freak, Sodium was convinced that exchanging electrons would benefit both elements.
In the end, the sodium gives its electrons to the fluorine to becomes an anion and their positive and negative charges attract forming an ionic bond -- Sodium Fluoride.
This is a non-metals only world!
O
N
Cl
Br
Why are they have so many electrons? Where is here?
Can anyone help me get rid of my electron?
UGH, NO WAY!
We don't even know which group you belongs to.
Get away from us!
P
F
Cl
Br
Na
S
Ar
Se
Thank you. Wow, you almost having a full valence shell..
I heard that you with you can get rid of your electron. I think we can exchange our electrons and I will be the anion who have the full valence shell and you can get rid of your electron.
Okay, it sounds to be a good idea.
Now, they form an ionic-bond which is NaF
WOW! I lose an electron and I feel so positive now!
+
-
We changed from atoms to ions and I gained an electron to become an anion
This is a world with all the non-metals gathers together. It's a nonmetal-only world, with all the different groups in it. Reactive metals in group 1 and some of elements in group 14-17, and the noble gases in group 18.In the 100 years of its existence, there has never exit an element that does not belong to the non-metals groups.
But one day, a metal who calls Sodium came into this unusual world.The element that is metallic disrupts the peace of this world.Nonmetals are curious about where this element comes from, they treat it very rudely and call she “the freak”.
All the non-metals began to squeeze out this stranger, and no one wants to exchange their electron with she. The nonmetalseven want to send she out of their world where they think it only belongs to nonmetals.
A non-metal calls Fluorine was willing to make friends with Sodium. Despite the objection of Fluorine’s family and friends, over time they were classified as the same categories — “the freak”.
Fluorine is the only element that shows a willingness to exchange electrons with Sodium. Although Sodium was a little shy at first because she also thinks of herself as a freak, Sodium was convinced that exchanging electrons would benefit both elements.
In the end, the sodium gives its electrons to the fluorine to becomes an anion and their positive and negative charges attract forming an ionic bond -- Sodium Fluoride.
This is a non-metals only world!
O
N
Cl
Br
Why are they have so many electrons? Where is here?
Can anyone help me get rid of my electron?
UGH, NO WAY!
We don't even know which group you belongs to.
Get away from us!
P
F
Cl
Br
Na
S
Ar
Se
Thank you. Wow, you almost having a full valence shell..
I heard that you with you can get rid of your electron. I think we can exchange our electrons and I will be the anion who have the full valence shell and you can get rid of your electron.
Okay, it sounds to be a good idea.
Now, they form an ionic-bond which is NaF
WOW! I lose an electron and I feel so positive now!
+
-
We changed from atoms to ions and I gained an electron to become an anion
Text z Príbehu
- O
- This is a non-metals only world!
- N
- Br
- Cl
- Why are they have so many electrons? Where is here?
- Na
- P
- F
- UGH, NO WAY!
- Cl
- Can anyone help me get rid of my electron?
- Br
- We don't even know which group you belongs to.
- Se
- Get away from us!
- S
- Ar
- This is a world with all the non-metals gathers together. It's a nonmetal-only world, with all the different groups in it. Reactive metals in group 1 and some of elements in group 14-17, and the noble gases in group 18. In the 100 years of its existence, there has never exit an element that does not belong to the non-metals groups.
- Hi, I am Fluorine, you are cute.
- Thank you. Wow, you almost having a full valence shell..
- I heard that you with you can get rid of your electron. I think we can exchange our electrons and I will be the anion who have the full valence shell and you can get rid of your electron.
- But one day, a metal who calls Sodium came into this unusual world. The element that is metallic disrupts the peace of this world. Nonmetals are curious about where this element comes from, they treat it very rudely and call she “the freak”.
- Okay, it sounds to be a good idea.
- All the non-metals began to squeeze out this stranger, and no one wants to exchange their electron with she. The nonmetals even want to send she out of their world where they think it only belongs to nonmetals.
- WOW! I lose an electron and I feel so positive now!
- +
- Now, they form an ionic-bond which is NaF
- We changed from atoms to ions and I gained an electron to become an anion
- -
- A non-metal calls Fluorine was willing to make friends with Sodium. Despite the objection of Fluorine’s family and friends, over time they were classified as the same categories — “the freak”.
- Fluorine is the only element that shows a willingness to exchange electrons with Sodium. Although Sodium was a little shy at first because she also thinks of herself as a freak, Sodium was convinced that exchanging electrons would benefit both elements.
- In the end, the sodium gives its electrons to the fluorine to becomes an anion and their positive and negative charges attract forming an ionic bond -- Sodium Fluoride.



