Ionic bonding comic strip
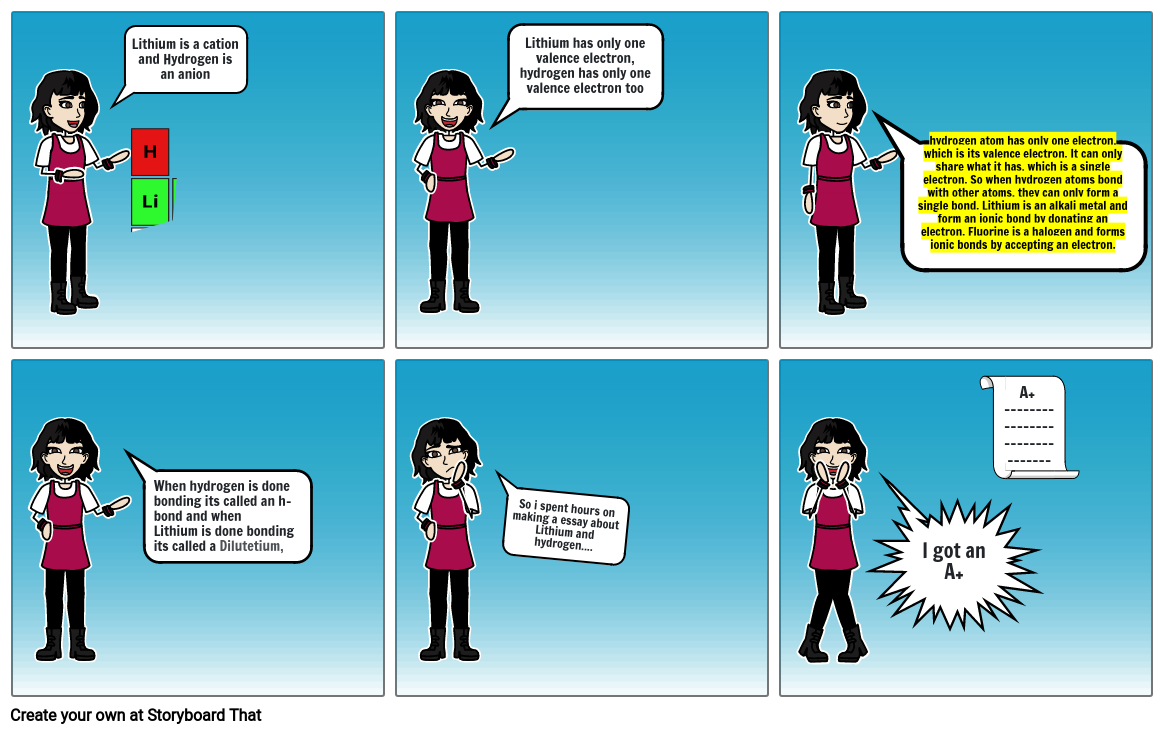
Lithium is a cation and Hydrogen is an anion
Lithium has only one valence electron, hydrogen has only one valence electron too
hydrogen atom has only one electron, which is its valence electron. It can only share what it has, which is a single electron. So when hydrogen atoms bond with other atoms, they can only form a single bond. Lithium is an alkali metal and form an ionic bond by donating an electron. Fluorine is a halogen and forms ionic bonds by accepting an electron.
When hydrogen is done bonding its called an h-bond and when Lithium is done bonding its called a Dilutetium,
So i spent hours on making a essay about Lithium and hydrogen....
I got an A+
A+
-------------------------------
Lithium is a cation and Hydrogen is an anion
Lithium has only one valence electron, hydrogen has only one valence electron too
hydrogen atom has only one electron, which is its valence electron. It can only share what it has, which is a single electron. So when hydrogen atoms bond with other atoms, they can only form a single bond. Lithium is an alkali metal and form an ionic bond by donating an electron. Fluorine is a halogen and forms ionic bonds by accepting an electron.
When hydrogen is done bonding its called an h-bond and when Lithium is done bonding its called a Dilutetium,
So i spent hours on making a essay about Lithium and hydrogen....
I got an A+
A+
-------------------------------
Lithium is a cation and Hydrogen is an anion
Lithium has only one valence electron, hydrogen has only one valence electron too
hydrogen atom has only one electron, which is its valence electron. It can only share what it has, which is a single electron. So when hydrogen atoms bond with other atoms, they can only form a single bond. Lithium is an alkali metal and form an ionic bond by donating an electron. Fluorine is a halogen and forms ionic bonds by accepting an electron.
When hydrogen is done bonding its called an h-bond and when Lithium is done bonding its called a Dilutetium,
So i spent hours on making a essay about Lithium and hydrogen....
I got an A+
A+
-------------------------------
Lithium is a cation and Hydrogen is an anion
Lithium has only one valence electron, hydrogen has only one valence electron too
hydrogen atom has only one electron, which is its valence electron. It can only share what it has, which is a single electron. So when hydrogen atoms bond with other atoms, they can only form a single bond. Lithium is an alkali metal and form an ionic bond by donating an electron. Fluorine is a halogen and forms ionic bonds by accepting an electron.
When hydrogen is done bonding its called an h-bond and when Lithium is done bonding its called a Dilutetium,
So i spent hours on making a essay about Lithium and hydrogen....
I got an A+
A+
-------------------------------
Lithium is a cation and Hydrogen is an anion
Lithium has only one valence electron, hydrogen has only one valence electron too
hydrogen atom has only one electron, which is its valence electron. It can only share what it has, which is a single electron. So when hydrogen atoms bond with other atoms, they can only form a single bond. Lithium is an alkali metal and form an ionic bond by donating an electron. Fluorine is a halogen and forms ionic bonds by accepting an electron.
When hydrogen is done bonding its called an h-bond and when Lithium is done bonding its called a Dilutetium,
So i spent hours on making a essay about Lithium and hydrogen....
I got an A+
A+
-------------------------------
Lithium is a cation and Hydrogen is an anion
Lithium has only one valence electron, hydrogen has only one valence electron too
hydrogen atom has only one electron, which is its valence electron. It can only share what it has, which is a single electron. So when hydrogen atoms bond with other atoms, they can only form a single bond. Lithium is an alkali metal and form an ionic bond by donating an electron. Fluorine is a halogen and forms ionic bonds by accepting an electron.
When hydrogen is done bonding its called an h-bond and when Lithium is done bonding its called a Dilutetium,
So i spent hours on making a essay about Lithium and hydrogen....
I got an A+
A+
-------------------------------
Lithium is a cation and Hydrogen is an anion
Lithium has only one valence electron, hydrogen has only one valence electron too
hydrogen atom has only one electron, which is its valence electron. It can only share what it has, which is a single electron. So when hydrogen atoms bond with other atoms, they can only form a single bond. Lithium is an alkali metal and form an ionic bond by donating an electron. Fluorine is a halogen and forms ionic bonds by accepting an electron.
When hydrogen is done bonding its called an h-bond and when Lithium is done bonding its called a Dilutetium,
So i spent hours on making a essay about Lithium and hydrogen....
I got an A+
A+
-------------------------------
Текст Раскадровки
- Lithium is a cation and Hydrogen is an anion
- Lithium has only one valence electron, hydrogen has only one valence electron too
- hydrogen atom has only one electron, which is its valence electron. It can only share what it has, which is a single electron. So when hydrogen atoms bond with other atoms, they can only form a single bond. Lithium is an alkali metal and form an ionic bond by donating an electron. Fluorine is a halogen and forms ionic bonds by accepting an electron.
- When hydrogen is done bonding its called an h-bond and when Lithium is done bonding its called a Dilutetium,
- So i spent hours on making a essay about Lithium and hydrogen....
- I got an A+
- A+ -------------------------------



