Importance of Acids, Bases and Salts
Текст Раскадровки
- Горка: 1
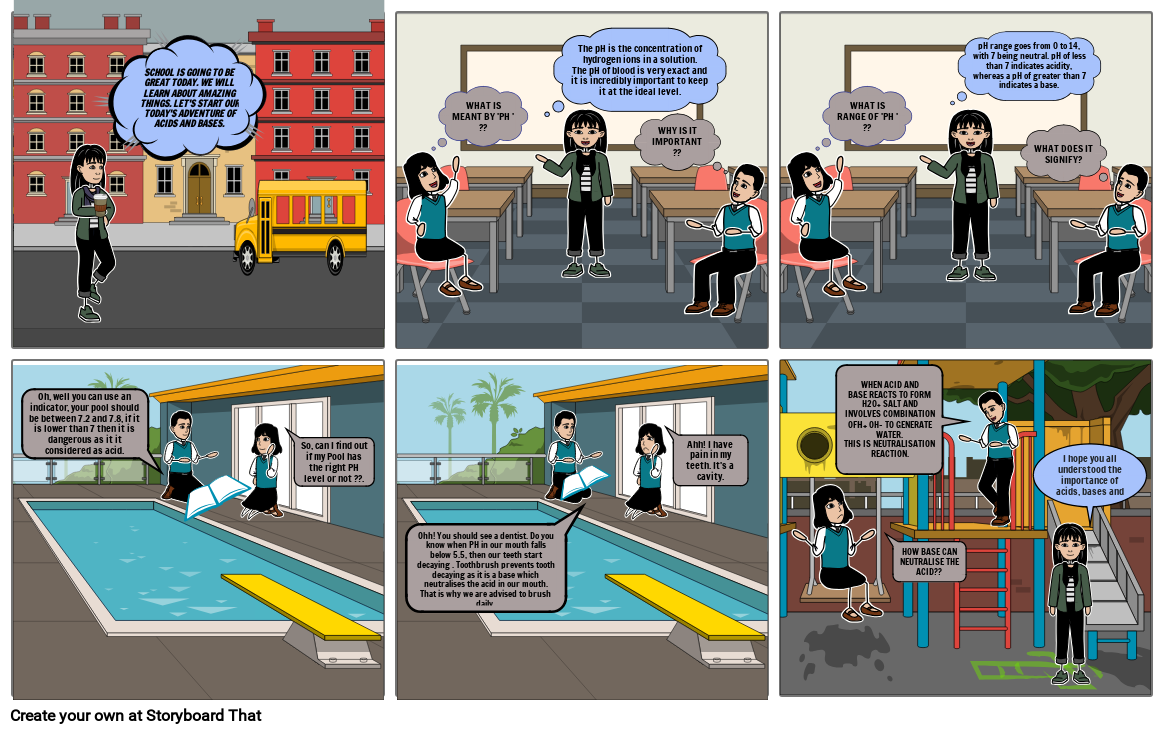
- SCHOOL IS GOING TO BE GREAT TODAY. WE WILL LEARN ABOUT AMAZING THINGS. LET'S START OUR TODAY'S ADVENTURE OF ACIDS AND BASES.
- Горка: 2
- The pH is the concentration of hydrogen ions in a solution.The pH of blood is very exact and it is incredibly important to keep it at the ideal level.
- WHAT IS MEANT BY 'PH ' ??
- WHY IS IT IMPORTANT ??
- Горка: 3
- pH range goes from 0 to 14, with 7 being neutral. pH of less than 7 indicates acidity, whereas a pH of greater than 7 indicates a base.
- WHAT IS RANGE OF 'PH ' ??
- WHAT DOES IT SIGNIFY?
- Горка: 4
- Oh, well you can use an indicator, your pool should be between 7.2 and 7.8, if it is lower than 7 then it is dangerous as it it considered as acid.
- So, can I find out if my Pool has the right PH level or not ??.
- Горка: 5
- Ahh! I have pain in my teeth. It's a cavity.
- Ohh! You should see a dentist. Do you know when PH in our mouth falls below 5.5, then our teeth start decaying . Toothbrush prevents tooth decaying as it is a base which neutralises the acid in our mouth. That is why we are advised to brush daily.
- Горка: 6
- WHEN ACID ANDBASE REACTS TO FORM H20+ SALT AND INVOLVES COMBINATION OFH+ OH- TO GENERATE WATER.THIS IS NEUTRALISATION REACTION.
- I hope you all understood the importance of acids, bases and salts.
- HOW BASE CAN NEUTRALISE THE ACID??
Создано более 30 миллионов раскадровок

