isotope notation
Текст Раскадровки
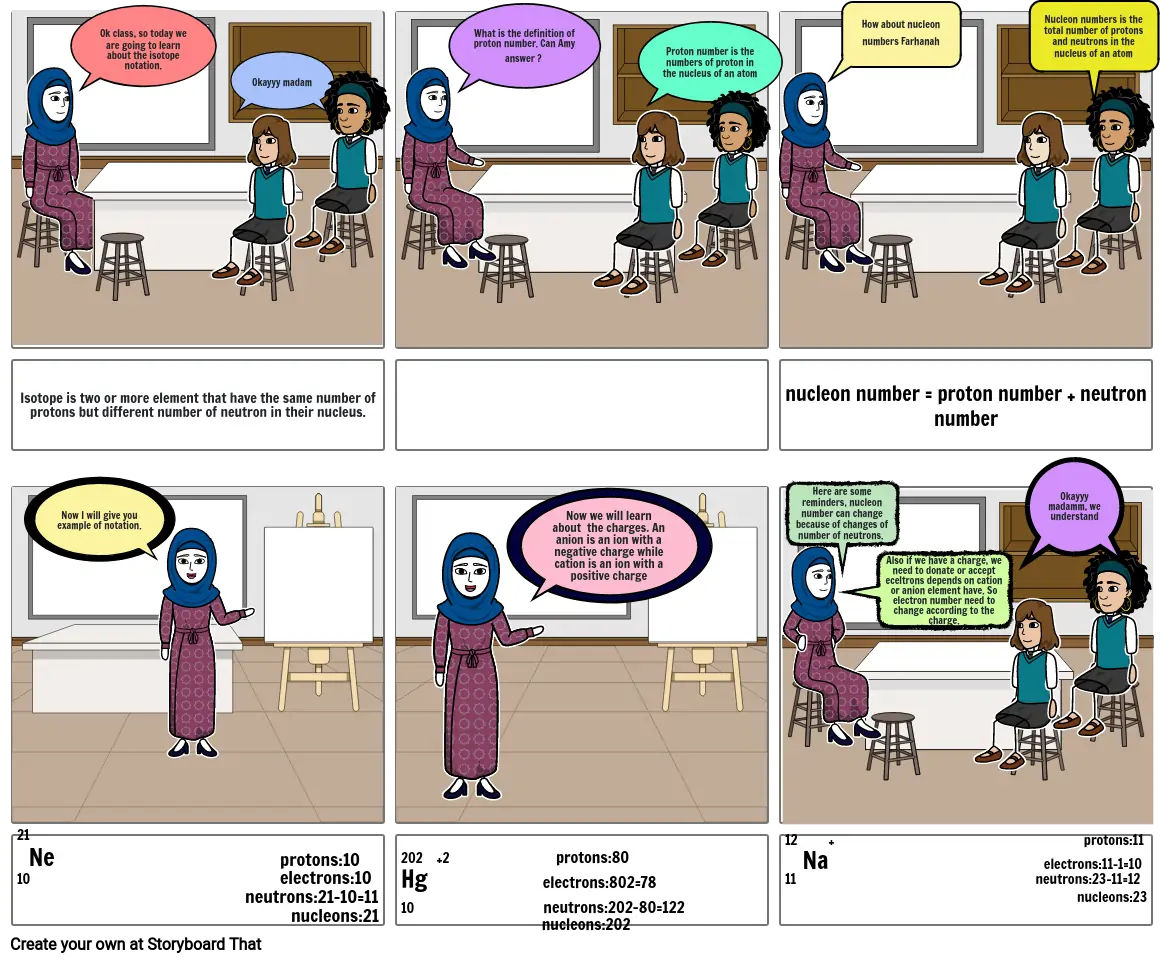
- Ok class, so today we are going to learn about the isotope notation.
- Okayyy madam
- What is the definition of proton number. Can Amy answer ?
- Proton number is the numbers of proton in the nucleus of an atom
- How about nucleon numbers Farhanah
- Nucleon numbers is the total number of protons and neutrons in the nucleus of an atom
- Isotope is two or more element that have the same number of protons but different number of neutron in their nucleus.
- Now I will give you example of notation.
-
- Now we will learn about the charges. An anion is an ion with a negative charge while cation is an ion with a positive charge
- nucleon number = proton number + neutron number
- Here are some reminders, nucleon number can change because of changes of number of neutrons.
- Also if we have a charge, we need to donate or accept eceltrons depends on cation or anion element have. So electron number need to change according to the charge.
- Okayyy madamm, we understand
- 21 Ne protons:10 10 electrons:10neutrons:21-10=11 nucleons:21
- 202 +2 protons:80Hg electrons:802=7810 neutrons:202-80=122 nucleons:202
- 12 + protons:11 Na electrons:11-1=1011 neutrons:23-11=12 nucleons:23
Создано более 30 миллионов раскадровок

