Chemistry Project
Texto do Storyboard
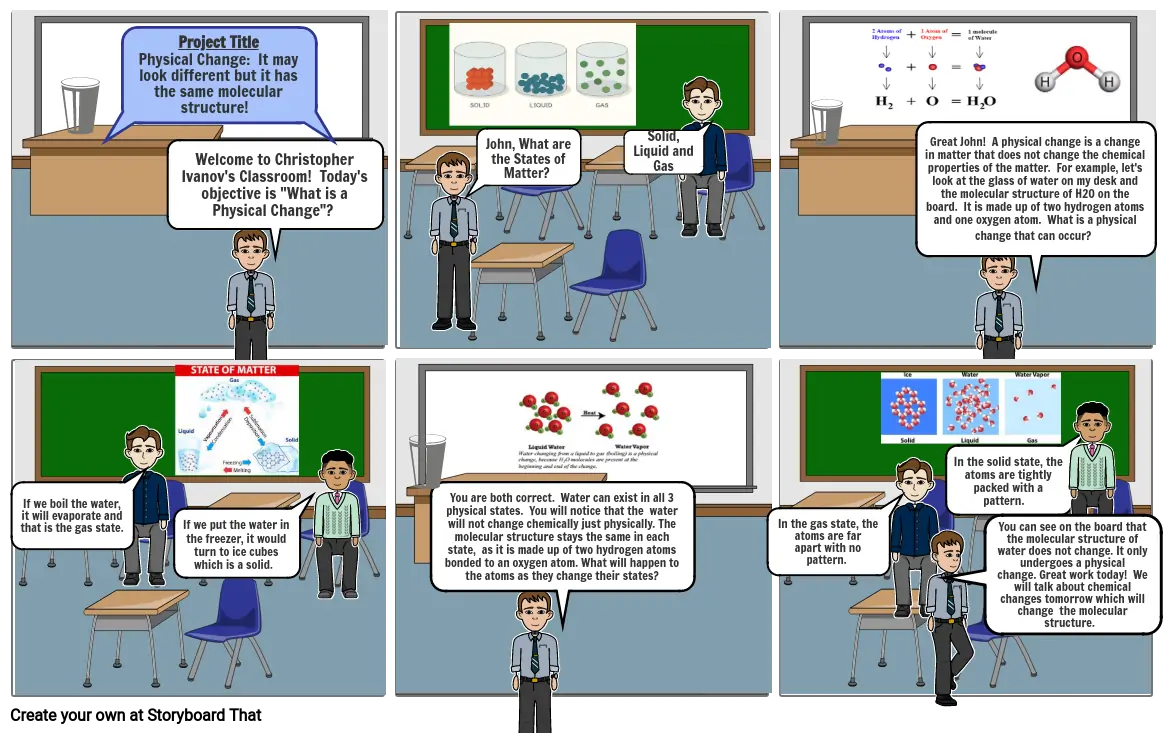
- Project Title Physical Change: It may look different but it has the same molecular structure!
- Welcome to Christopher Ivanov's Classroom! Today's objective is "What is a Physical Change"?
- John, What are the States of Matter?
- Solid, Liquid and Gas
- Great John! A physical change is a change in matter that does not change the chemical properties of the matter. For example, let's look at the glass of water on my desk and the molecular structure of H20 on the board. It is made up of two hydrogen atoms and one oxygen atom. What is a physical change that can occur?
- If we boil the water, it will evaporate and that is the gas state.
- If we put the water in the freezer, it would turn to ice cubes which is a solid.
- You are both correct. Water can exist in all 3 physical states. You will notice that the water will not change chemically just physically. The molecular structure stays the same in each state, as it is made up of two hydrogen atoms bonded to an oxygen atom. What will happen to the atoms as they change their states?
- In the gas state, the atoms are far apart with no pattern.
- You can see on the board that the molecular structure of water does not change. It only undergoes a physical change. Great work today! We will talk about chemical changes tomorrow which will change the molecular structure.
- In the solid state, the atoms are tightly packed with a pattern.
Mais de 30 milhões de storyboards criados

