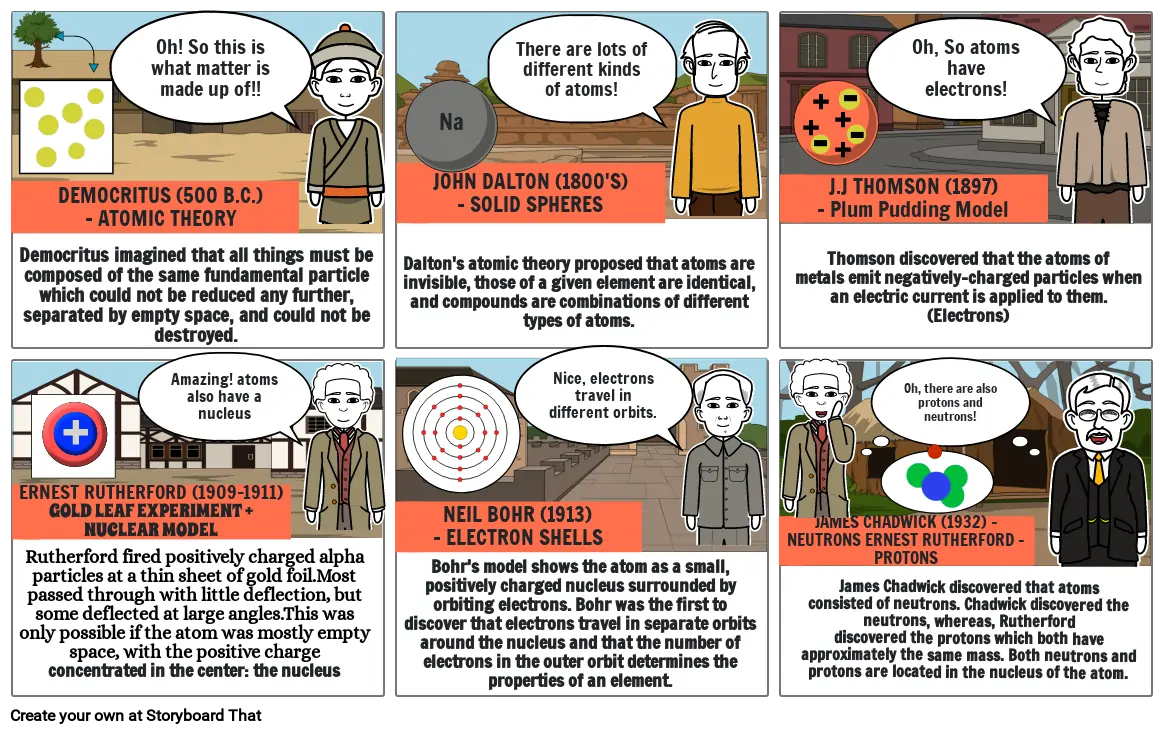
HISTORY OF THE ATOM
Texto do Storyboard
- DEMOCRITUS (500 B.C.)- ATOMIC THEORY
- Democritus imagined that all things must be composed of the same fundamental particle which could not be reduced any further, separated by empty space, and could not be destroyed.
- Oh! So this is what matter is made up of!!
- Dalton's atomic theory proposed that atoms are invisible, those of a given element are identical, and compounds are combinations of different types of atoms.
- JOHN DALTON (1800'S)- SOLID SPHERES
- Na
- There are lots of different kinds of atoms!
- Nice, electrons travel in different orbits.
- Thomson discovered that the atoms of metals emit negatively-charged particles when an electric current is applied to them. (Electrons)
- J.J THOMSON (1897)- Plum Pudding Model
- Oh, So atoms have electrons!
- ERNEST RUTHERFORD (1909-1911)GOLD LEAF EXPERIMENT + NUCLEAR MODEL
- Rutherford fired positively charged alpha particles at a thin sheet of gold foil.Most passed through with little deflection, but some deflected at large angles.This was only possible if the atom was mostly empty space, with the positive charge concentrated in the center: the nucleus
- Amazing! atoms also have a nucleus
- Bohr's model shows the atom as a small, positively charged nucleus surrounded by orbiting electrons. Bohr was the first to discover that electrons travel in separate orbits around the nucleus and that the number of electrons in the outer orbit determines the properties of an element.
- NEIL BOHR (1913)- ELECTRON SHELLS
- James Chadwick discovered that atoms consisted of neutrons. Chadwick discovered the neutrons, whereas, Rutherford discovered the protons which both have approximately the same mass. Both neutrons and protons are located in the nucleus of the atom.
- JAMES CHADWICK (1932) - NEUTRONS ERNEST RUTHERFORD - PROTONS
- Oh, there are also protons and neutrons!
Mais de 30 milhões de storyboards criados

