Bhor Model
Tekst Storyboardowy
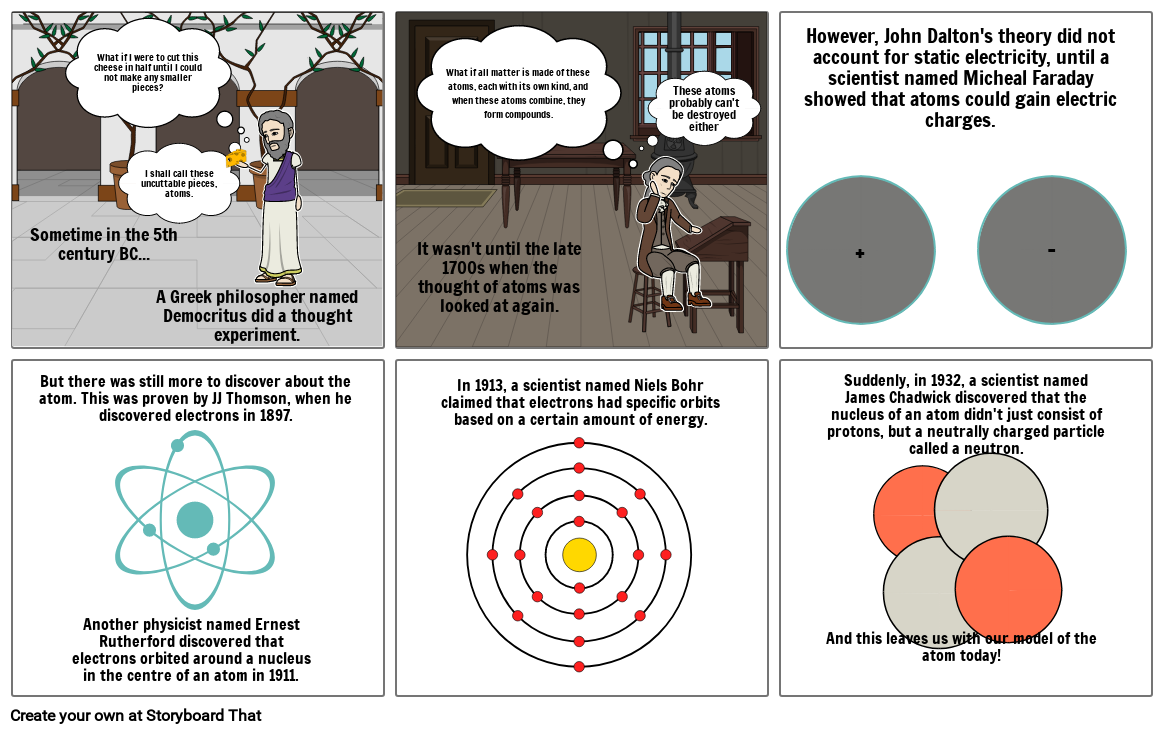
- Sometime in the 5th century BC...
- What if I were to cut this cheese in half until I could not make any smaller pieces?
- I shall call these uncuttable pieces, atoms.
- A Greek philosopher named Democritus did a thought experiment.
- It wasn't until the late 1700s when the thought of atoms was looked at again.
- What if all matter is made of these atoms, each with its own kind, and when these atoms combine, they form compounds.
- These atoms probably can't be destroyed either
- +
- However, John Dalton's theory did not account for static electricity, until a scientist named Micheal Faraday showed that atoms could gain electric charges.
- -
- But there was still more to discover about the atom. This was proven by JJ Thomson, when he discovered electrons in 1897.
- Another physicist named Ernest Rutherford discovered that electrons orbited around a nucleus in the centre of an atom in 1911.
- In 1913, a scientist named Niels Bohr claimed that electrons had specific orbits based on a certain amount of energy.
- And this leaves us with our model of the atom today!
- Suddenly, in 1932, a scientist named James Chadwick discovered that the nucleus of an atom didn't just consist of protons, but a neutrally charged particle called a neutron.
Utworzono ponad 30 milionów scenorysów

