Unknown Story
Storyboard Tekst
- Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn
-
- Zn2+ Zn2+ Zn2+ Zn2+ Zn2+ Zn2+ Zn2+ Zn2+ Zn2+ Zn2+ Zn2+ Zn2+ Zn2+ Zn2+ Zn2+ Zn2+ Zn2+
- Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn Zn
- Cu Cu Cu Cu Cu Cu Cu Cu
- Cu
- Zn
- Zn2+
- Zn2+
- K+
- OH-
- OH-
- K+
- K+
- OH-
- K+
- OH-
- Zn2+
- Zn2+
- Zn2+
- OH-
- K+
- Zn2+
- K+
- K+
- Cu
- Zn2+
- Zn
- OH-
- OH-
- OH-
- K+
- K+
- Zn2+
- OH-
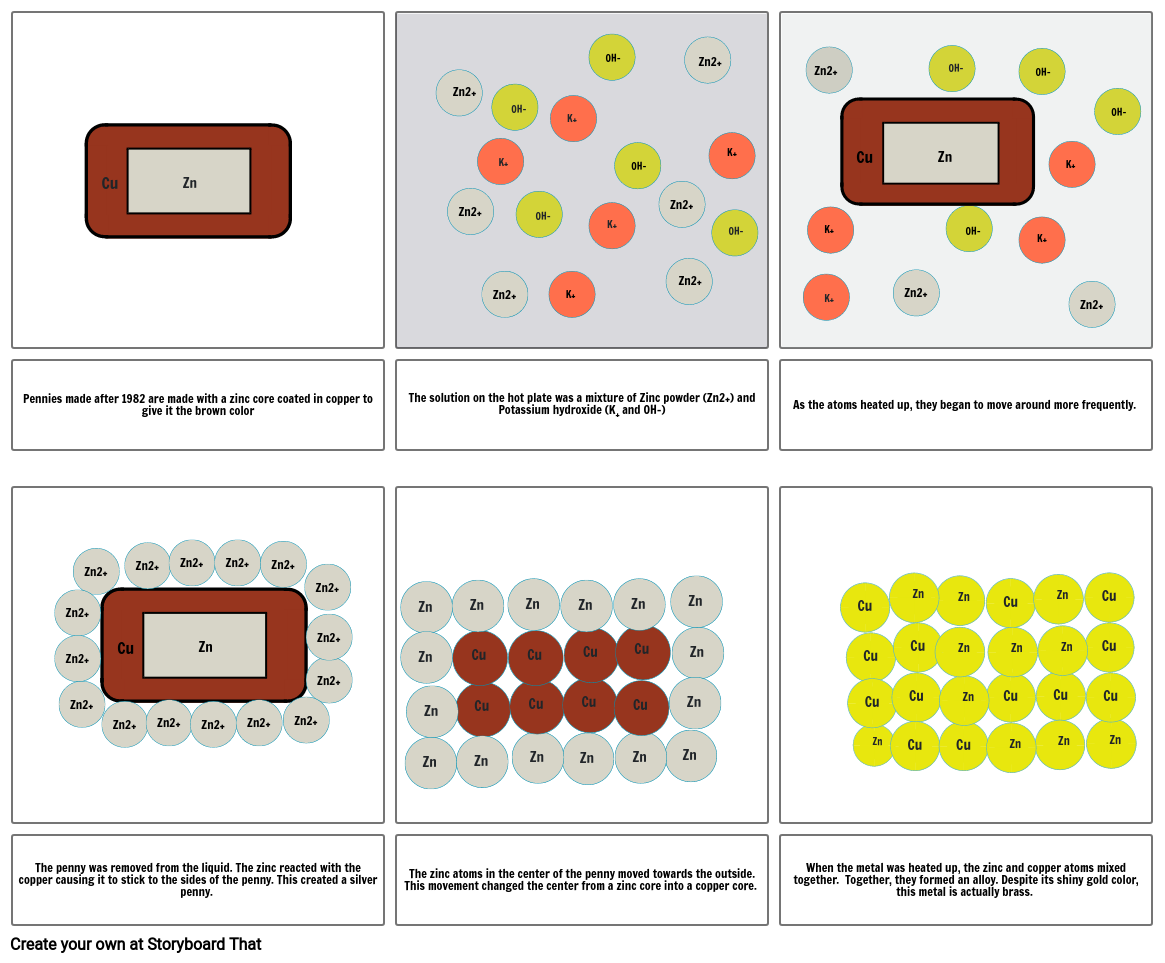
- Pennies made after 1982 are made with a zinc core coated in copper to give it the brown color
- Cu
- Zn
- The solution on the hot plate was a mixture of Zinc powder (Zn2+) and Potassium hydroxide (K+ and OH-)
- As the atoms heated up, they began to move around more frequently.
- Cu
- Cu
- Cu
- Cu
- Cu
- Cu
- Cu
- Cu
- Cu
- Cu
- Cu
- The penny was removed from the liquid. The zinc reacted with the copper causing it to stick to the sides of the penny. This created a silver penny.
- The zinc atoms in the center of the penny moved towards the outside. This movement changed the center from a zinc core into a copper core.
- When the metal was heated up, the zinc and copper atoms mixed together. Together, they formed an alloy. Despite its shiny gold color, this metal is actually brass.
- Cu
- Cu
Over 30 millioner storyboards opprettet

