Atomic Theory Comic Strip #1
Storyboard Tekst
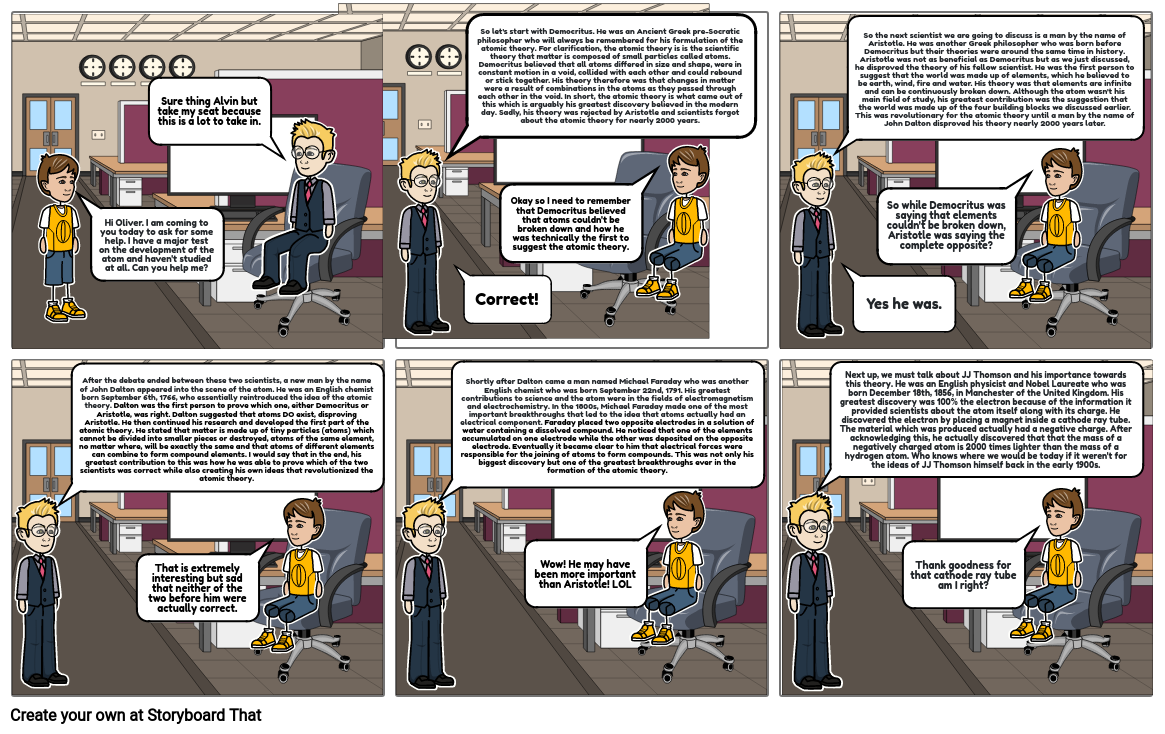
- Hi Oliver. I am coming to you today to ask for some help. I have a major test on the development of the atom and haven't studied at all. Can you help me?
- Sure thing Alvin but take my seat because this is a lot to take in.
- So let's start with Democritus. He was an Ancient Greek pre-Socratic philosopher who will always be remembered for his formulation of the atomic theory. For clarification, the atomic theory is is the scientific theory that matter is composed of small particles called atoms. Democritus believed that all atoms differed in size and shape, were in constant motion in a void, collided with each other and could rebound or stick together. His theory therefore was that changes in matter were a result of combinations in the atoms as they passed through each other in the void. In short, the atomic theory is what came out of this which is arguably his greatest discovery believed in the modern day. Sadly, his theory was rejected by Aristotle and scientists forgot about the atomic theory for nearly 2000 years.
- Correct!
- Okay so I need to remember that Democritus believed that atoms couldn't be broken down and how he was technically the first to suggest the atomic theory.
- So the next scientist we are going to discuss is a man by the name of Aristotle. He was another Greek philosopher who was born before Democritus but their theories were around the same time in history. Aristotle was not as beneficial as Democritus but as we just discussed, he disproved the theory of his fellow scientist. He was the first person to suggest that the world was made up of elements, which he believed to be earth, wind, fire and water. His theory was that elements are infinite and can be continuously broken down. Although the atom wasn't his main field of study, his greatest contribution was the suggestion that the world was made up of the four building blocks we discussed earlier. This was revolutionary for the atomic theory until a man by the name of John Dalton disproved his theory nearly 2000 years later.
- Yes he was.
- So while Democritus was saying that elements couldn't be broken down, Aristotle was saying the complete opposite?
- After the debate ended between these two scientists, a new man by the name of John Dalton appeared into the scene of the atom. He was an English chemist born September 6th, 1766, who essentially reintroduced the idea of the atomic theory. Dalton was the first person to prove which one, either Democritus or Aristotle, was right. Dalton suggested that atoms DO exist, disproving Aristotle. He then continued his research and developed the first part of the atomic theory. He stated that matter is made up of tiny particles (atoms) which cannot be divided into smaller pieces or destroyed, atoms of the same element, no matter where, will be exactly the same and that atoms of different elements can combine to form compound elements. I would say that in the end, his greatest contribution to this was how he was able to prove which of the two scientists was correct while also creating his own ideas that revolutionized the atomic theory.
- That is extremely interesting but sad that neither of the two before him were actually correct.
- Shortly after Dalton came a man named Michael Faraday who was another English chemist who was born September 22nd, 1791. His greatest contributions to science and the atom were in the fields of electromagnetism and electrochemistry. In the 1800s, Michael Faraday made one of the most important breakthroughs that led to the idea that atoms actually had an electrical component. Faraday placed two opposite electrodes in a solution of water containing a dissolved compound. He noticed that one of the elements accumulated on one electrode while the other was deposited on the opposite electrode. Eventually it became clear to him that electrical forces were responsible for the joining of atoms to form compounds. This was not only his biggest discovery but one of the greatest breakthroughs ever in the formation of the atomic theory.
- Wow! He may have been more important than Aristotle! LOL
- Next up, we must talk about JJ Thomson and his importance towards this theory. He was an English physicist and Nobel Laureate who was born December 18th, 1856, in Manchester of the United Kingdom. His greatest discovery was 100% the electron because of the information it provided scientists about the atom itself along with its charge. He discovered the electron by placing a magnet inside a cathode ray tube. The material which was produced actually had a negative charge. After acknowledging this, he actually discovered that that the mass of a negatively charged atom is 2000 times lighter than the mass of a hydrogen atom. Who knows where we would be today if it weren't for the ideas of JJ Thomson himself back in the early 1900s.
- Thank goodness for that cathode ray tube am I right?
Meer dan 30 miljoen storyboards gemaakt

